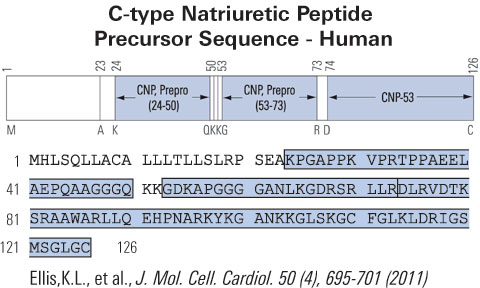
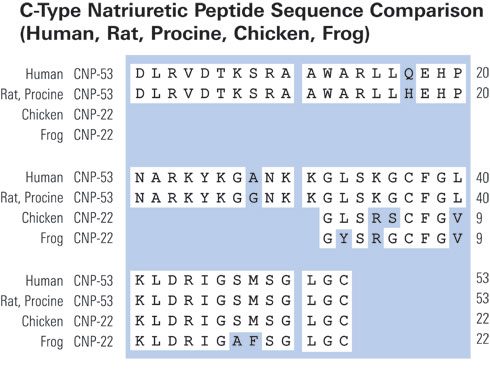
   |
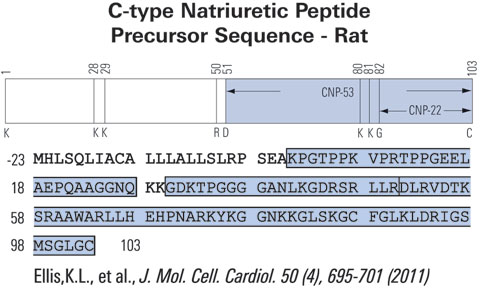
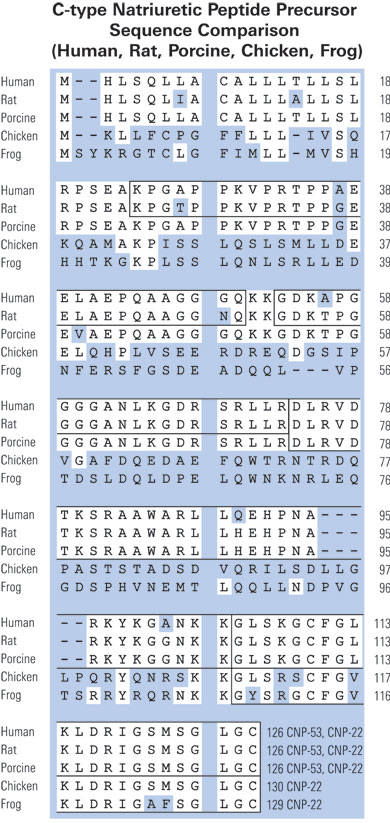 |
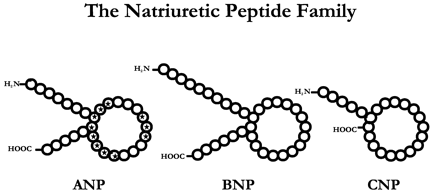
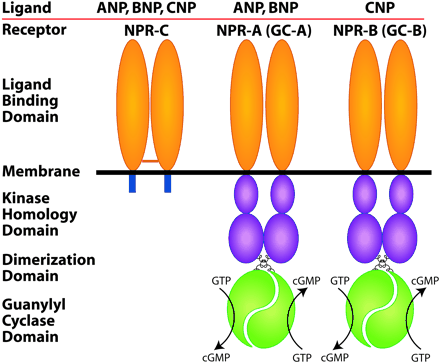
The natriuretic peptides (NP) are a family of three polypeptide hormones termed atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP). ANP regulates a variety of physiological parameters by interacting with its receptors present on the plasma membrane. These are of three subtypes NPR-A, NPR-B, and NPR-C. NPR-A and NPR-B are guanylyl cyclase receptors, whereas NPR-C is non-guanylyl cyclase receptor and is coupled to adenylyl cyclase inhibition or phospholipase C activation through inhibitory guanine nucleotide regulatory protein (Gi). ANP, BNP, CNP, as well as C-ANP (4-23), a ring deleted peptide that specifically interacts with NPR-C receptor inhibit adenylyl cyclase activity through Gi protein. Unlike other G-protein-coupled receptors, NPR-C receptors have a single transmembrane domain and a short cytoplasmic domain of 37 amino acids, which has a structural specificity like those of other single transmembrane domain receptors. A 37 amino acid cytoplasmic peptide is sufficient to inhibit adenylyl cyclase activity with an apparent Ki similar to that of ANP (99-126) or C-ANP (4-23). In addition, C-ANP (4-23) also stimulates phosphatidyl inositol (PI) turnover in vascular smooth muscle cells (VSMC) which is attenuated by dbcAMP and cAMP-stimulatory agonists, suggesting that NPR-C receptor-mediated inhibition of adenylyl cyclase and resultant decreased levels of cAMP may be responsible for NPR-C-mediated stimulation of PI turnover. Furthermore, the activation of NPR-C receptor by C-ANP (4-23) and CNP inhibits the mitogen-activated protein kinase activity stimulated by endothelin-3, platelet-derived growth factor, phorbol-12 myristate 13-acetate, suggesting that NPR-C receptor might also be coupled to other signal transduction system or that there may be an interaction of the NPR-C receptor and some other signaling pathways. In this review article, NPR-C receptor coupling to different signaling pathways and their regulation will be discussed.
Anand-srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides. 2005;26(6):1044-5
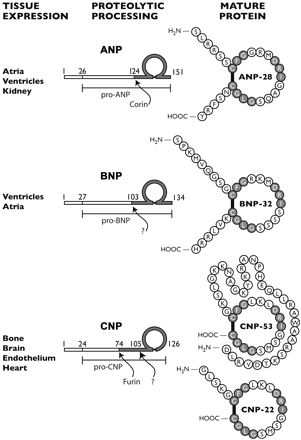
The C-type natriuretic (CNP) peptide signals through the type B natriuretic peptide receptor (NPR-B) in vascular smooth muscle cells to activate the particulate guanylyl cyclase activity intrinsic to that receptor and raise cellular cyclic GMP levels. In the present study, we demonstrate that CNP down-regulates the expression of this receptor leading to a reduction in NPR-B activity. Pretreatment of rat aortic smooth muscle cells with CNP reduces NPR-B activity, NPR-B protein levels, NPR2 (NPR-B gene) mRNA levels, and NPR2 promoter activity. The decrease in NPR2 promoter activity is dependent on DNA sequence present between -441 and -134 relative to the transcription start site. The reduction in NPR2 gene expression appears to operate through generation of cyclic GMP. 8-Bromo cyclic GMP, a membrane-permeable cyclic GMP analog, reduced NPR2 mRNA levels and NPR2 promoter activity. Atrial natriuretic peptide, which signals through the type A natriuretic peptide receptor (NPR-A) to increase cyclic GMP levels in these cells, also reduced NPR-B mRNA levels and inhibited NPR-B promoter activity; however, this inhibition was not additive with that produced by CNP, implying that the two ligands traffic over a common signal transduction pathway. This report provides the first documentation that CNP is capable of autoregulating the expression of its cognate receptor.
Rahmutula D, Gardner DG. C-type natriuretic Peptide down-regulates expression of its cognate receptor in rat aortic smooth muscle cells. Endocrinology. 2005;146(11):4968-74
C-Type natriuretic peptide (CNP) is a member of the family of natriuretic peptides with vasodilatory properties, and is produced and secreted by endothelial cells. It seems to play a central role in the paracrine vasomotor control of tone and to be important in several clinical conditions characterized by endothelial dysfunction. We evaluated the analytical performance of a commercially available radioimmunoassay for CNP (CNP-22 (Human, Rat, Mouse) RIA Kit, Phoenix Pharmaceuticals, Inc.) after a preliminary extraction with Sep-Pak C18. Its analytical reliability was checked by determination of CNP plasma levels in healthy subjects (n=23) and in patients with different diseases, likely characterized by endothelial dysfunction, such as chronic heart failure (n=133) and cirrhosis (n s 84). The extraction yield was 78+/-3%. Accuracy of the radiommunological determination was evaluated by dilution (45-370 [mu]L of extracted plasma) and recovery tests (>80%). Between- and within-assay variabilities were <=10% and analytical sensitivity was 0.41+/-0.015 pg/tube. Plasma CNP in patients with chronic heart failure and with cirrhosis was significantly raised compared to controls (p<0.0001 and p=0.001, respectively). The sensitivity, accuracy and variability levels of the method proposed for CNP assay was suitable for reliable detection of changes in CNP plasma levels in the clinical setting.
Del ry S, Maltinti M, Emdin M, Passino C, Catapano G, Giannessi D. Radioimmunoassay for plasma C-type natriuretic peptide determination: a methodological evaluation. Clin Chem Lab Med. 2005;43(6):641-5
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 012-03 | CNP-22 (Human, Rat, Mouse, Porcine, Bovine, Monkey) | 200 µg | $97 |
| RK-012-03 | CNP-22 (Human, Rat, Mouse, Porcine, Bovine, Monkey) - RIA Kit | 125 tubes | $926 |
| EK-012-03 | CNP-22 (Human, Rat, Mouse, Porcine, Bovine, Monkey) - EIA Kit | 96 wells | $570 |
| RKU-012-03 | CNP-22 (Human, Rat, Mouse, Porcine, Bovine, Monkey) - Ultra-Sensitive RIA Kit | 125 tubes | $771 |
| 012-05 | CNP-53 (Rat, Porcine) | 20 µg | $287 |
| CEK-012-03 | CNP-22 (Human, Rat, Mouse, Porcine, Bovine, Monkey) - Chemiluminescent EIA Kit | 96 wells | $624 |
| FEK-012-03 | CNP-22 (Human, Rat, Mouse, Porcine, Bovine, Monkey) - Fluorescent EIA Kit | 96 wells | $624 |
| EKE-012-03 | CNP-22 (Human, Rat, Mouse, Porcine, Bovine, Monkey) - Extraction-Free EIA Kit | 96 wells | $649 |
| 012-09 | CNP-53 (Human) | 100 µg | $470 |
| G-012-03 | CNP-22 (Human, Rat, Mouse, Porcine, Bovine, Monkey) - Purified IgG Antibody | 200 µg | $571 |
Social Network Confirmation