Abstract: Although accumulating evidence has shown the important function of long non-coding RNAs (lncRNAs) in tumor progression and chemotherapy resistance, the role of lncRNA DLEU1 in regulating proliferation, invasion, and chemoresistance of bladder cancer (BCA) cells remains largely unknown. Here, we found that DLEU1 was upregulated in BLCA tissues and BCA patients with high DLEU1 expression exhibited a shorter survival time. Furthermore, mechanistic analysis and functional assays validated that DLEU1 induced cell proliferation, invasion, and cisplatin resistance of BCA cells by de-repressing the expression of HS3ST3B1 through sponging miR-99b. Low miR-99b and high HS3ST3B1 levels were correlated with worse prognosis in patients with BCA. Ectopic expression of HS3ST3B1 or inhibition of miR-99b reversed DLEU1 knockdown-mediated suppression of cell proliferation, invasion, and cisplatin resistance. Thus, our study revealed a novel role for the DLEU1/miR-99b/HS3ST3B1 axis in regulating proliferation, invasion, and cisplatin resistance of BCA cells.
Li Y, Shi B, Dong F, Zhu X, Liu B, Liu Y. Long non-coding rna dleu1 promotes cell proliferation, invasion, and confers cisplatin resistance in bladder cancer by regulating the mir-99b/hs3st3b1 axis. Front Genet. 2019;10:280.
Background: Accumulating evidences show that long noncoding RNAs (lncRNA) play essential roles in the development and progression of various malignancies. However, their functions remains poorly understood and many lncRNAs have not been defined in colorectal cancer (CRC). In this study, we investigated the role of DLEU1 in CRC.
Methods: Quantitative real-time PCR was used to detect the expression of DLEU1 and survival analysis was adopted to explore the association between DLEU1 expression and the prognosis of CRC patients. CRC cells were stably transfected with lentivirus approach and cell proliferation, migration, invasion and cell apoptosis, as well as tumorigenesis in nude mice were performed to assess the effects of DLEU1 in BCa. Biotin-coupled probe pull down assay, RNA immunoprecipitation and Fluorescence in situ hybridization assays were conducted to confirm the relationship between DLEU1 and SMARCA1.
Results: Here we revealed that DLEU1 was crucial for activation of KPNA3 by recruiting SMARCA1, an essential subunit of the NURF chromatin remodeling complex, in CRC. DLEU1 was indispensable for the deposition of SMARCA1 at the promoter of KPNA3 gene. Increased expression of DLEU1 and KPNA3 was observed in human CRC tissues. And higher expression of DLEU1 or KPNA3 in patients indicates lower survival rate and poorer prognosis. DLEU1 knockdown remarkably inhibited CRC cell proliferation, migration and invasion in vitro and in vivo while overexpressing KPNA3 in the meantime reversed it.
Conclusions: Our results identify DLEU1 as a key regulator by a novel DLEU1/SMARCA1/KPNA3 axis in CRC development and progression, which may provide a potential biomarker and therapeutic target for the management of CRC.
Liu T, Han Z, Li H, Zhu Y, Sun Z, Zhu A. LncRNA DLEU1 contributes to colorectal cancer progression via activation of KPNA3. Mol Cancer. 2018;17(1):118
Abstract: Recent studies have shown that long noncoding RNAs (lncRNAs) have pivotal roles in human malignancies, although their significance in oral squamous cell carcinoma (OSCC) is not fully understood. In the present study, we identified lncRNAs functionally associated with OSCC. By analyzing RNA-seq datasets obtained from primary head and neck squamous cell carcinoma (HNSCC), we identified 15 lncRNAs aberrantly expressed in cancer tissues. We then validated their expression in 18 OSCC cell lines using qRT-PCR and identified 6 lncRNAs frequently overexpressed in OSCC. Among those, we found that knocking down DLEU1 (deleted in lymphocytic leukemia 1) strongly suppressed OSCC cell proliferation. DLEU1 knockdown also suppressed migration, invasion, and xenograft formation by OSCC cells, which is suggestive of its oncogenic functionality. Microarray analysis revealed that DLEU1 knockdown significantly affects expression of a number of cancer-related genes in OSCC cells, including HAS3, CD44, and TP63, suggesting that DLEU1 regulates HA-CD44 signaling. Expression of DLEU1 was elevated in 71% of primary OSCC tissues, and high DLEU1 expression was associated with shorter overall survival of HNSCC patients. These data suggest that elevated DLEU1 expression contributes to OSCC development, and that DLEU1 may be a useful therapeutic target in OSCC.
Nishiyama K, Maruyama R, Niinuma T, et al. Screening for long noncoding RNAs associated with oral squamous cell carcinoma reveals the potentially oncogenic actions of DLEU1. Cell Death Dis. 2018;9(8):826.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
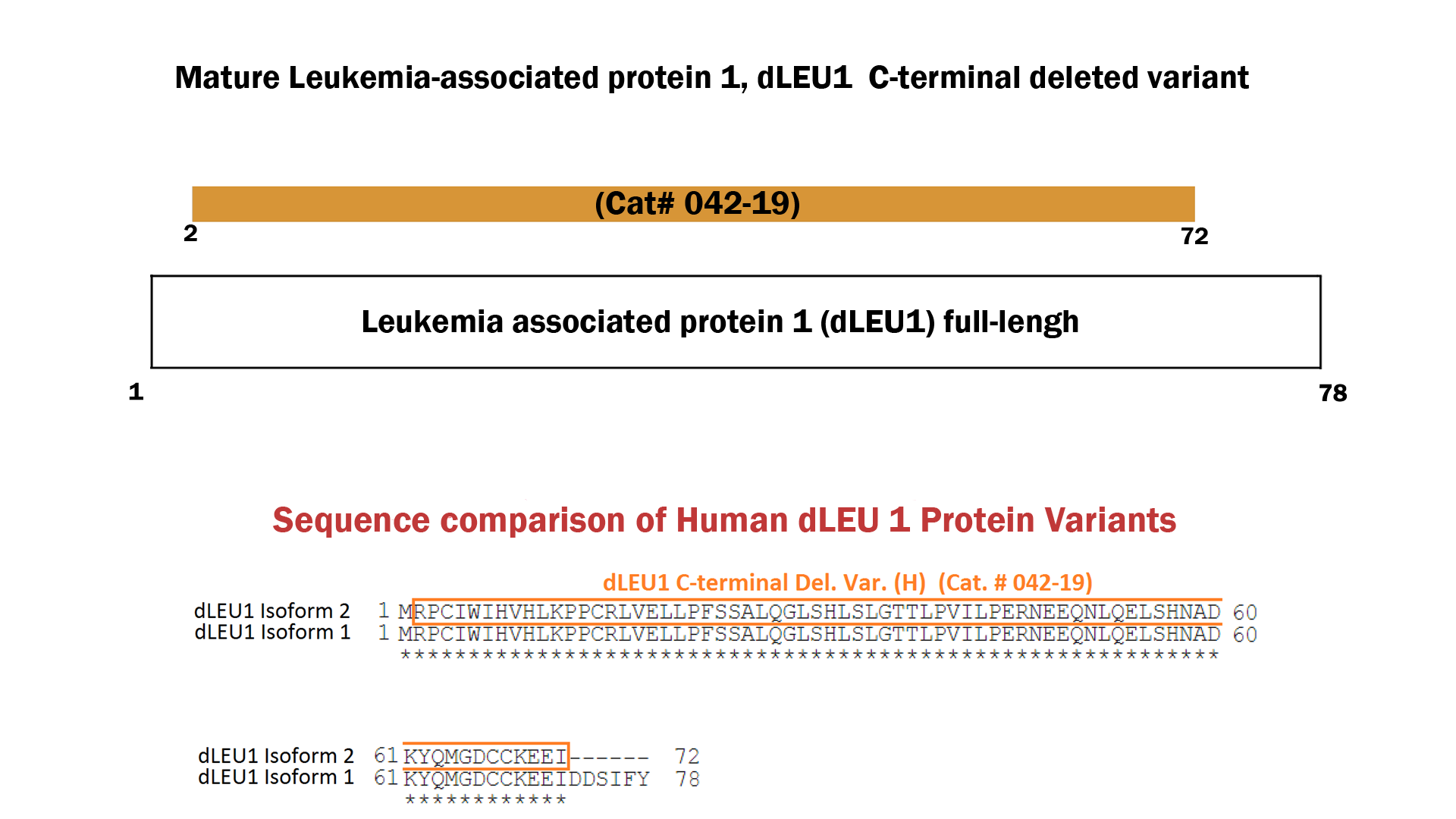
| 042-19 | dLEU1 C-terminal Deleted Variant (Human) | 100ug | $595 |
Social Network Confirmation