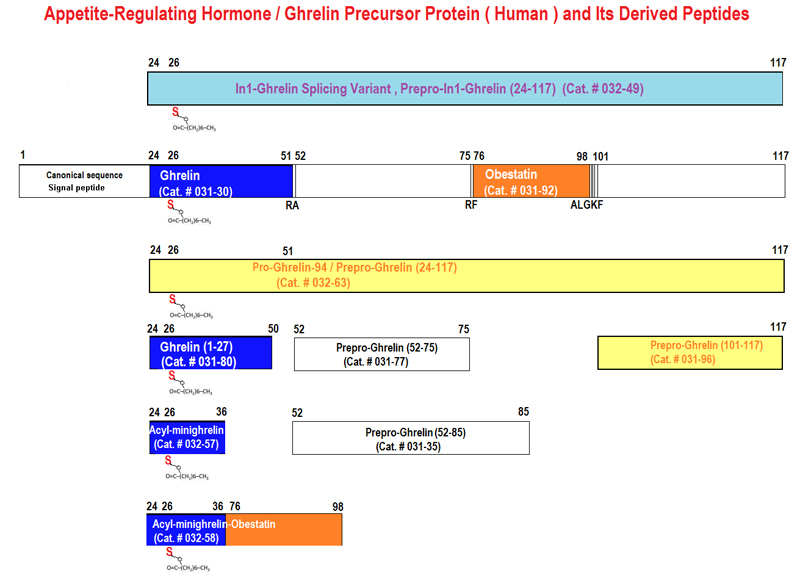
Ghrelin and obestatin are two gastrointestinal peptides, derived from a common precursor. Expression of both peptides have been found in breast cancer tissue and ghrelin has been associated with breast cancer development. Ghrelin expression is associated with longer survival in women diagnosed with invasive and node negative breast cancer. The clinical implications of the peptide expression in male breast cancer are unclear. The aim of this study was to investigate the role and potential clinical value of ghrelin and obestatin in male breast cancer. A tissue microarray of invasive male breast cancer specimens from 197 patients was immunostained with antibodies versus the two peptides. The expression of the peptides was correlated to previously known prognostic factors in breast cancer and to the outcome. No strong correlations were found between ghrelin or obestatin expression and other known prognostic factors. Only ghrelin expression was statistically significantly correlated to breast cancer-specific survival (HR 0.39, 95% CI 0.18-0.83) in univariate analyses and in multivariate models, adjusted for tumor size and node status (HR 0.38, 95% CI 0.17-0.87). HR for obestatin was 0.38 (95% CI 0.11-1.24). Ghrelin is a potential prognostic factor for breast cancer death in male breast cancer. Patients with tumors expressing ghrelin have a 2.5-fold lower risk for breast cancer death than those lacking ghrelin expression. Drugs targeting ghrelin are currently being investigated in clinical studies treating metabolic or nutritional disorders. Ghrelin should be further evaluated in forthcoming studies as a prognostic marker with the aim to be included in decision algorithms.Grönberg M, Nilsson C, Markholm I, et al. Ghrelin expression is associated with a favorable outcome in male breast cancer. Sci Rep. 2018;8(1):13586.
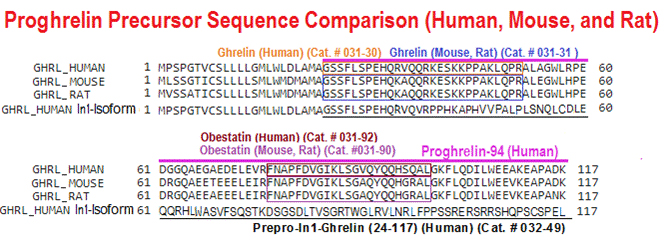
BACKGROUND: The Ghrelin-system is a complex, pleiotropic family composed of several peptides, including native-ghrelin and its In1-ghrelin splicing variant, and receptors (GHSR 1a/b), which are dysregulated in various endocrine-related tumors, where they associate to pathophysiological features, but the presence, functional role, and mechanisms of actions of In1-ghrelin splicing variant in prostate-cancer (PCa), is completely unexplored. Herein, we aimed to determine the presence of key ghrelin-system components (native-ghrelin, In1-ghrelin, GHSR1a/1b) and their potential pathophysiological role in prostate cancer (PCa).
METHODS: In1-ghrelin and native-ghrelin expression was evaluated by qPCR in prostate tissues from patients with high PCa-risk (n = 52; fresh-tumoral biopsies), and healthy-prostates (n = 12; from cystoprostatectomies) and correlated with clinical parameters using Spearman-test. In addition, In1-ghrelin and native-ghrelin was measured in plasma from an additional cohort of PCa-patients with different risk levels (n = 30) and control-healthy patients (n = 20). In vivo functional (proliferation/migration) and mechanistic (gene expression/signaling-pathways) assays were performed in PCa-cell lines in response to In1-ghrelin and native-ghrelin treatment, overexpression and/or silencing. Finally, tumor progression was monitored in nude-mice injected with PCa-cells overexpressing In1-ghrelin, native-ghrelin and empty vector (control).
RESULTS: In1-ghrelin, but not native-ghrelin, was overexpressed in high-risk PCa-samples compared to normal-prostate (NP), and this expression correlated with that of PSA. Conversely, GHSR1a/1b expression was virtually absent. Remarkably, plasmatic In1-ghrelin, but not native-ghrelin, levels were also higher in PCa-patients compared to healthy-controls. Furthermore, In1-ghrelin treatment/overexpression, and to a much lesser extent native-ghrelin, increased aggressiveness features (cell-proliferation, migration and PSA secretion) of NP and PCa cells. Consistently, nude-mice injected with PC-3-cells stably-transfected with In1-ghrelin, but not native-ghrelin, presented larger tumors. These effects were likely mediated by ERK1/2-signaling activation and involved altered expression of key oncogenes/tumor suppressor genes. Finally, In1-ghrelin silencing reduced cell-proliferation and PSA secretion from PCa cells.
CONCLUSIONS: Altogether, our results indicate that In1-ghrelin levels (in tissue) and circulating levels (in plasma) are increased in PCa where it can regulate key pathophysiological processes, thus suggesting that In1-ghrelin may represent a novel biomarker and a new therapeutic target in PCa.Hormaechea-agulla D, Gahete MD, Jiménez-vacas JM, et al. The oncogenic role of the In1-ghrelin splicing variant in prostate cancer aggressiveness. Mol Cancer. 2017;16(1):146.
Ghrelin is a 28-amino-acid hormone derived from the endoproteolytic processing of its prehormone proghrelin. Although ghrelin has been reported to regulate food intake and body weight, it is still unknown whether proghrelin exercises any biological function. Here we show that recombinant proghrelin alters food intake and energy metabolism in mice. After intraperitoneal administration of recombinant proghrelin (100 nmol/kg body wt), cumulative food intake was significantly increased at days 1, 2, and 3 (6 +/- 0.3, 13 +/- 0.5, and 20 +/- 0.8 g vs. 5 +/- 0.2, 10 +/- 0.2, and 16 +/- 0.3 g of the control mice receiving normal saline, respectively, n = 6, P < 0.05). Twelve-hour cumulative food intake in the light photo period in mice treated with proghrelin increased significantly relative to the control (2.1 +/- 0.04 vs. 1.3 +/- 0.2 g, n = 6, P < 0.05). No change in 12-h cumulative food intake in the dark photo period was observed between mice treated with proghrelin and vehicle (4.2 +/- 0.6 vs. 4.3 +/- 0.6 g, n = 6, P > 0.05). This is associated with a decrease in body weight (0.42 +/- 0.04 g) for mice treated with proghrelin, whereas control animals gained body weight (0.31 +/- 0.04 g). Mice treated with proghrelin demonstrate a significant decrease in respiratory quotient, indicating an increase in fat consumption. Recombinant proghrelin is functionally active with effects on food intake and energy metabolism.Zhang W, Majumder A, Wu X, Mulholland MW. Regulation of food intake and body weight by recombinant proghrelin. Am J Physiol Endocrinol Metab. 2009;297(6):E1269-75.
We provide the first evidence for the existence in human plasma of peptides derived from the 66 carboxyl-terminal amino acids of pro-ghrelin (C-ghrelin). C-ghrelin immunoreactivity in plasma was higher than ghrelin, and did not significantly correlate with body mass index in normal health. In patients with myocardial infarction, plasma levels of both ghrelin and C-ghrelin were significantly decreased (approximately 30%, P<0.05), whereas in patients with heart failure, C-ghrelin levels were significantly elevated (approximately 32%, P<0.05) compared with controls. HPLC coupled with RIA showed circulating C-ghrelin to be primarily of low molecular weight (M(r) approximately 3500), but in chronic heart failure, a higher molecular weight form (M(r) approximately 7500) is also present. This is the first evidence for potential circulating hormones derived from the carboxyl terminus of pro-ghrelin and for their modulation in cardiovascular diseases.Pemberton C, Wimalasena P, Yandle T, Soule S, Richards M. C-terminal pro-ghrelin peptides are present in the human circulation. Biochem Biophys Res Commun. 2003;310(2):567-73.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| EK-032-63 | Proghrelin-94 (Human) - EIA Kit | 96 Wells | $570 |
| RK-032-63 | Proghrelin-94 (Human) - RIA Kit | 125 Tubes | $926 |
Social Network Confirmation