The vast majority of ovarian cancer-related deaths are caused by metastatic dissemination of tumor cells, resulting in subsequent organ failure. However, despite our increased understanding of the physiological processes involved in tumor metastasis, there are no clinically approved drugs that have made a major impact in increasing the overall survival of patients with advanced, metastatic ovarian cancer. We identified prosaposin (psap) as a potent inhibitor of tumor metastasis, which acts via stimulation of p53 and the antitumorigenic protein thrombospondin-1 (TSP-1) in bone marrow-derived cells that are recruited to metastatic sites. We report that more than 97% of human serous ovarian tumors tested express CD36, the receptor that mediates the proapoptotic activity of TSP-1. Accordingly, we sought to determine whether a peptide derived from psap would be effective in treating this form of ovarian cancer. To that end, we developed a cyclic peptide with drug-like properties derived from the active sequence in psap. The cyclic psap peptide promoted tumor regression in a patient-derived tumor xenograft model of metastatic ovarian cancer. Thus, we hypothesize that a therapeutic agent based on this psap peptide would have efficacy in treating patients with metastatic ovarian cancer.
Wang S, Blois A, El Rayes T et al., Sci Transl Med. 2016 Mar 9;8(329):329ra34. doi: 10.1126/scitranslmed.aad5653.
Esophageal squamous cell carcinoma (ESCC) is one of the most common malignant neoplasms worldwide. Patients are often diagnosed at advanced stages with poor prognosis due to the absence of obvious early symptoms. Here, we applied a high-throughput serum peptidome analysis to identify circulating;peptide markers of ESCC. Weak cationic exchange magnetic beads coupled to matrix-assisted laser desorption / ionization time-of-flight mass spectrometry was used for two-stage proteotypic peptide profiling in complex serum samples collected from 477 cancer patients and healthy controls. We established a genetic algorithm model containing three significantly differentially expressed peptides at 1,925.5, 2,950.6 and 5,900.0 Da with a sensitivity and specificity of 97.00% and 95.92% in the training set and 97.03% and 100.00% in the validation set, respectively. The model's diagnostic capability was significantly better than SCC-Ag and Cyfra 21-1, especially for early stage ESCC, with an achieved sensitivity of 96.94%. Subsequently, these peptides were identified as fragments of AHSG, TSP1 and FGA by linear ion trap-orbitrap hybrid tandem mass spectrometry. Notably, increased tissue and serum levels of TSP1 in ESCC were verified and correlated with disease progression. In addition, tissue TSP1 was an independent poor prognostic factor in ESCC. In conclusion, the newly established circulating peptide panel and identified proteins could serve as potential biomarkers for the early detection and diagnosis of ESCC. Nevertheless, a larger cohort will be required for further unequivocal validation of their clinical application.
Jia K, Li W, Wang F et al., Oncotarget. 2016 Mar 16. doi: 10.18632/oncotarget.8123. [Epub ahead of print]
Orphan G protein-coupled receptors (GPCRs) represent a largely untapped resource for the treatment of a variety of diseases, despite sophisticated advances in drug discovery. Two promising orphan GPCRs are the endothelin B receptor-like proteins, GPR37 [ET(B)R-LP, Pael-R] and GPR37L1 [ET(B)R-LP-2]. Originally identified through searches for homologs of endothelin and bombesin receptors, neither GPR37 nor GPR37L1 were found to bind endothelins or related peptides. Instead, GPR37 was proposed to be activated by head activator (HA) and both GPR37 and GPR37L1 have been linked to the neuropeptides prosaposin and prosaptide, although these pairings are yet to be universally acknowledged. Both orphan GPCRs are widely expressed in the brain, where GPR37 has received the most attention for its link to Parkinson's disease and parkinsonism, while GPR37L1 deletion leads to precocious cerebellar development and hypertension. In this review, the existing pharmacology and physiology of GPR37 and GPR37L1 is discussed and the potential therapeutic benefits of targeting these receptors are explored.
Smith NJ. Front Pharmacol. 2015 Nov 17;6:275. doi: 10.3389/fphar.2015.00275. eCollection 2015.
Neutrophil elastase (NE) and cathepsin G (CG) contribute to intracellular microbial killing but, if left unchecked and released extracellularly, promote tissue damage. Conversely, mechanisms that constrain neutrophil serine protease activity protect against tissue damage but may have the untoward effect of disabling the microbial killing arsenal. The host elaborates thrombospondin-1 (TSP-1), a matricellular protein released during inflammation, but its role during neutrophil activation following microbial pathogen challenge remains uncertain. Mice deficient in TSP-1 (thbs1(-/-)) showed enhanced lung bacterial clearance, reduced splenic dissemination, and increased survival compared with wild-type (WT) controls during intrapulmonary Klebsiella pneumoniae infection. More effective pathogen containment was associated with reduced burden of inflammation in thbs1(-/-) mouse lungs compared with WT controls. Lung NE activity was increased in thbs1(-/-) mice following K. pneumoniae challenge, and thbs1(-/-) neutrophils showed enhanced intracellular microbial killing that was abrogated with recombinant TSP-1 administration or WT serum. Thbs1(-/-) neutrophils exhibited enhanced NE and CG enzymatic activity, and a peptide corresponding to amino-acid residues 793-801 within the type-III repeat domain of TSP-1 bridled neutrophil proteolytic function and microbial killing in vitro. Thus, TSP-1 restrains proteolytic action during neutrophilic inflammation elicited by K. pneumoniae, providing a mechanism that may regulate the microbial killing arsenal.
Zhao Y, Olonisakin TF, Xiong Z et al., Mucosal Immunol. 2015 Jul;8(4):896-905. doi: 10.1038/mi.2014.120. Epub 2014 Dec 10.
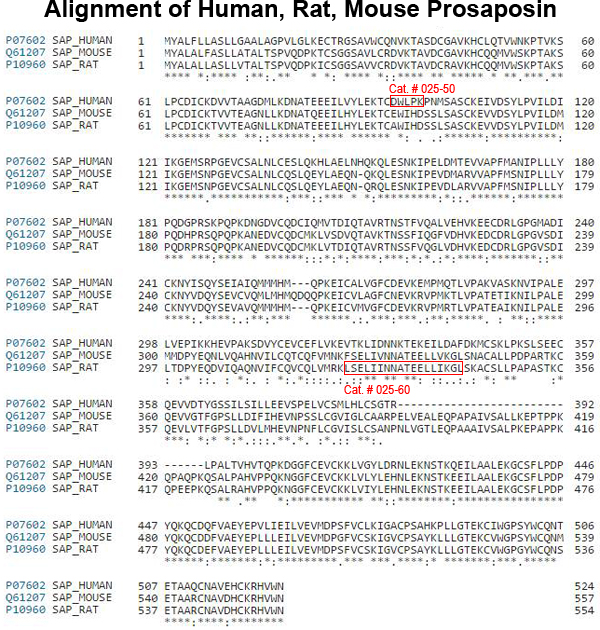
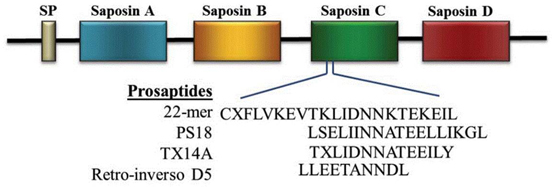
Four sphingolipid activator proteins (i.e., saposins A-D) are synthesized from a single precursor protein, prosaposin (PS), which exerts exogenous neurotrophic effects in vivo and in vitro. Kainic acid (KA) injection in rodents is a good model in which to study neurotrophic factor elevation; PS and its mRNA are increased in neurons and the choroid plexus in this animal model. An 18-mer peptide (LSELIINNATEELLIKGL; PS18) derived from the PS neurotrophic region prevents neuronal damage after ischemia, and PS18 is a potent candidate molecule for use in alleviating ischemia-induced learning disabilities and neuronal loss. KA is a glutamate analog that stimulates excitatory neurotransmitter release and induces ischemia-like neuronal degeneration; it has been used to define mechanisms involved in neurodegeneration and neuroprotection. In the present study, we demonstrate that a subcutaneous injection of 0.2 and 2.0 mg/kg PS18 significantly improved behavioral deficits of Wistar rats (n = 6 per group), and enhanced the survival of hippocampal and cortical neurons against neurotoxicity induced by 12 mg/kg KA compared with control animals. PS18 significantly protected hippocampal synapses against KA-induced destruction. To evaluate the extent of PS18- and KA-induced effects in these hippocampal regions, we performed histological evaluations using semithin sections stained with toluidine blue, as well as ordinal sections stained with hematoxylin and eosin. We revealed a distinctive feature of KA-induced brain injury, which reportedly mimics ischemia, but affects a much wider area than ischemia-induced injury: KA induced neuronal degeneration not only in the CA1 region, where neurons degenerate following ischemia, but also in the CA2, CA3, and CA4 hippocampal regions.
Nabeka H, Shimokawa T, Doihara T et al., PLoS One. 2015 May 18;10(5):e0126856. doi: 10.1371/journal.pone.0126856. eCollection 2015.
Prosaposin (also known as SGP-1) is an intriguing multifunctional protein that plays roles both intracellularly, as a regulator of lysosomal enzyme function, and extracellularly, as a secreted factor with neuroprotective and glioprotective effects. Following secretion, prosaposin can undergo endocytosis via an interaction with the low-density lipoprotein-related receptor 1 (LRP1). The ability of secreted prosaposin to promote protective effects in the nervous system is known to involve activation of G proteins, and the orphan G protein-coupled receptors GPR37 and GPR37L1 have recently been shown to mediate signaling induced by both prosaposin and a fragment of prosaposin known as prosaptide. In this review, we describe recent advances in our understanding of prosaposin, its receptors and their importance in the nervous system.
Meyer RC, Giddens MM, Coleman BM, Hall RA, Brain Res. 2014 Oct 17;1585:1-12. doi: 10.1016/j.brainres.2014.08.022.
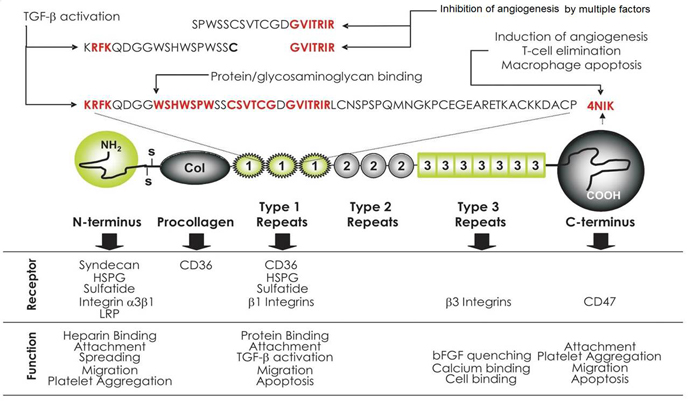
Thrombospondin-1 is the first and most studied naturally occurring protein inhibitor of angiogenesis. Its characteristic multi-domain structure determines thrombospondin-1 divergent functions, which include but are not limited to the regulation of angiogenesis. Below we overview the structural determinants and receptors expressed on the endothelial and other cell types, that are at the root of thrombospondin-1 striking ability to block neovascularization. We specifically emphasize thrombospondin-1 direct apoptotic action on the remodeling vascular endothelium and summarize current knowledge of its pro-apoptotic signaling and transcriptional networks. Further, we provide comprehensive survey of thethrombospondin-based anti-angiogenic strategies with special focus on the combination treatments. We convincingly illustrate how precise knowledge of the pro-apoptotic events and intermediates elicited by thrombospondin in the vascular endothelial cells facilitates the design of the most effective treatment combinations, where the efficacy of thrombospondin-derived compounds is maximized by the partner drug(s) ("complementation" strategies) and provide examples of such fine-tuning of the thrombospondin-based anti-angiogenic treatments.
Mirochnik Y, Kwiatek A, Volpert OV. Curr Drug Targets. 2008 Oct;9(10):851-62.
OBJECTIVE: To evaluate the effect of a thrombospondin 1 (TSP1)-derived peptide on inflammation and angiogenesis in an animal model of erosive arthritis and to assess the relationship between TSP1 and connective tissue growth factor (CTGF) in the pathophysiology of rheumatoid arthritis.
METHODS: Erosive arthritis in Lewis rats was induced by peptidoglycan-polysaccharide (PG-PS). Animals were divided into four groups: (1) negative control and groups receiving, (2) no treatment, (3) treatment with a TSP1-derived peptide, and (4) treatment with a scrambled peptide. Samples obtained from ankle joint, spleen and liver were studied using histology, histomorphometry, immunohistochemistry and RT-PCR.
RESULTS: Histological data indicated that the TSP1-derived peptide treatment decreased neovascularization, leukocyte infiltration and thickening of the synovial lining of the joint, and reduced granuloma formation in the spleen and liver when compared to control groups. Higher concentrations of CTGF and TSP1 proteins were observed in the affected areas of animals which did not receive TSP1-derived peptide treatment. Also, immunofluorescence and RT-PCR analyses showed an increase in CTGF protein expression and regulation, respectively, in the tissues of untreated animals when compared to the TSP1-derived peptide treated animals. By immunofluorescence, TSP1 expression was decreased in the TSP1-derived peptide treated animals. Moreover, macrophage/monocyte-specific staining revealed a decrease in cell infiltration in the articular tissue of the TSP1-derived peptide treated animals.
CONCLUSION: Both inflammation and angiogenesis were decreased after TSP1-derived peptide treatment indicating a potential pathway by which TSP1 interaction with neutrophils induces CTGF in RA affected tissues.
Rico MC, Castaneda JL, Manns JM et al., J Cell Physiol. 2007 May;211(2):504-12.
The mechanisms regulating T lymphocyte migration within the extracellular matrix are not understood. We show in this study that the thrombospondin-1 binding site of calreticulin, spanning aa 19-32, is a major triggering factor for T cell motility and migration within a three-dimensional collagen type 1 matrix, and that exogenous motogenic factors such as chemokines can stimulate migration via a calreticulin-thrombospondin-1 pathway. Endogenous calreticulin binding to the N-terminal domain of endogenous thrombospondin-1 elicited a motogenic signal to the T cells through the C-terminal domain of thrombospondin-1 and its cell surface receptor integrin-associated protein (CD47). Our data further revealed that thrombospondin-1 was expressed on the cell surface with a high turnover, and that PI3K and the Janus family of tyrosine kinases were required for T cell motility mediated through calreticulin, thrombospondin-1, and CD47. These results unveil an autocrine mechanism of calreticulin-thrombospondin-1-CD47 interaction for the control of T cell motility and migration within three-dimensional extracellular matrix substrata.Li SS, Forslöw A, Sundqvist KG et al., J Immunol. 2005 Jan 15;174(2):654-61.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 025-60 | Prosapsin (324-341) / PS18 (Rat) | 200 µg | $242 |
| 025-61 | Prosaptide / TX14 (A) | 500 µg | $124 |
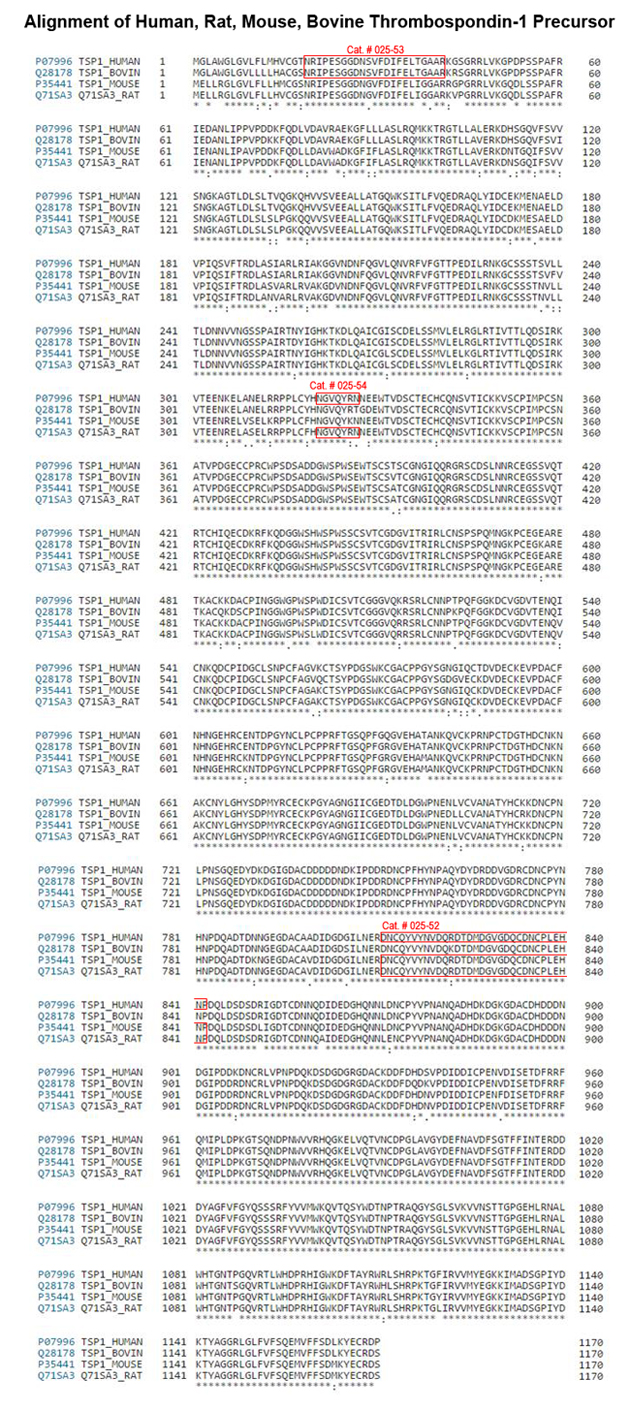
| 025-52 | TSP-1 Type-3 D793-P824 (Human, Rat, Mouse) | 100 µg | $247 |
| 025-51 | TSP-Hep1 / A1 (Human) | 200 µg | $280 |
| 025-53 | Thrombospondin-1 (1-23) (Human, Bovine) | 100 µg | $210 |
| 025-55 | [Lys1015, Lys1024]-Thrombospondin-1 (1015-1024) / 4KN1 (Human, Mouse, Bovine) | 500 µg | $147 |
| 025-54 | Thrombospondin-1 precursor (321-327) (Human, Rat) | 500 µg | $174 |
Social Network Confirmation