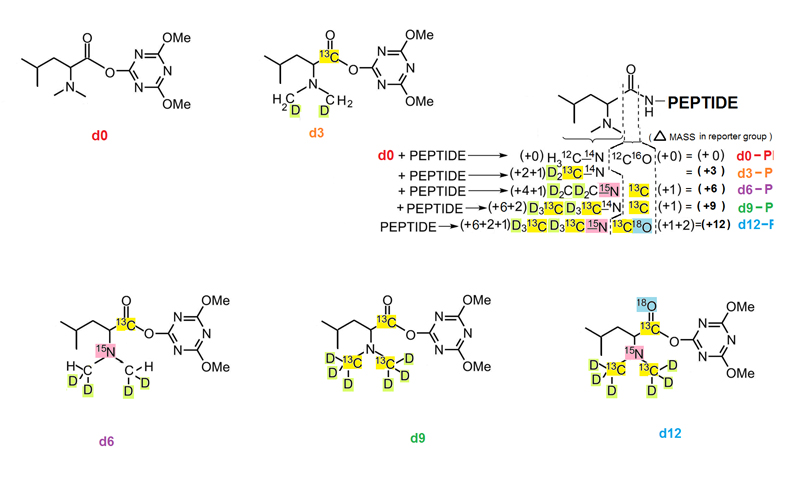
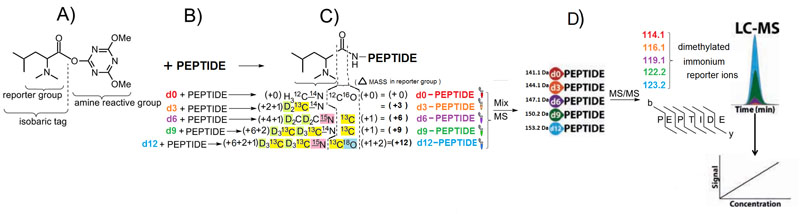
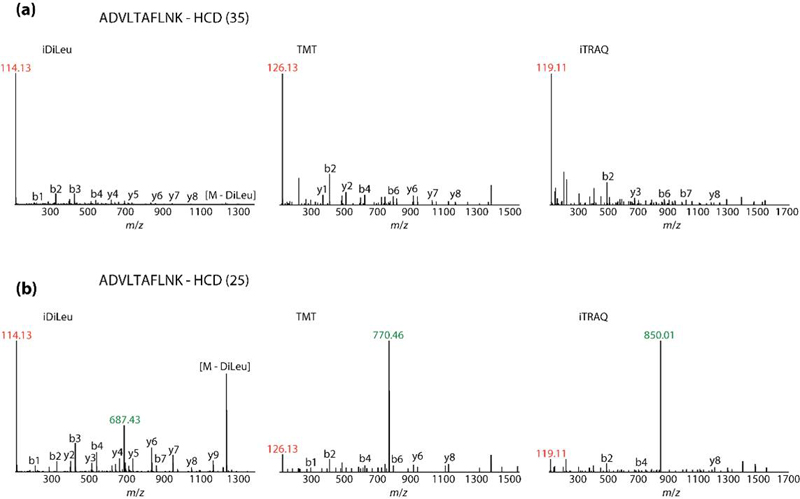
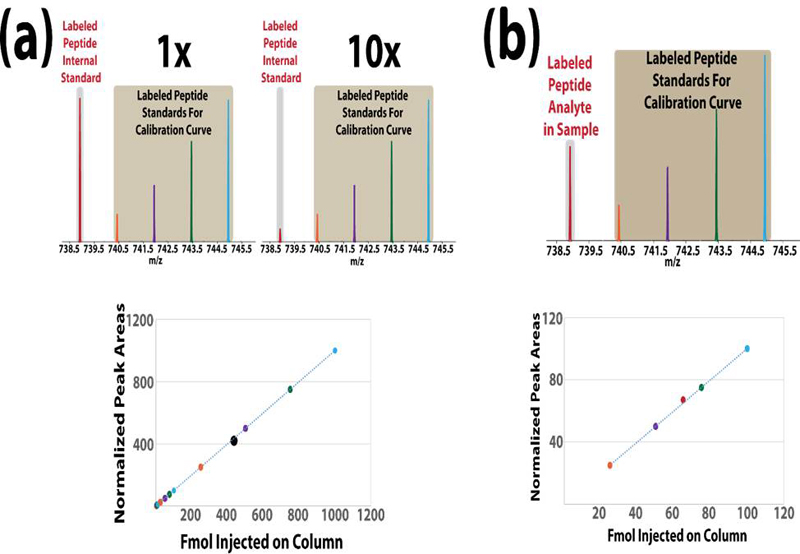
Absolute quantification of protein targets using liquid chromatography-mass spectrometry (LC-MS) is a key component of candidate biomarker validation. One popular method combines multiple reaction monitoring (MRM) using a triple quadrupole instrument with stable isotope-labeled standards (SIS) for absolute quantification (AQUA). LC-MRM AQUA assays are sensitive and specific, but they are also expensive because of the cost of synthesizing stable isotope peptide standards. While the chemical modification approach using mass differential tags for relative and absolute quantification (mTRAQ) represents a more economical approach when quantifying large numbers of peptides, these reagents are costly and still suffer from lower throughput because only two concentration values per peptide can be obtained in a single LC-MS run. Here, we have developed and applied a set of five novel mass difference reagents, isotopic N,N-dimethyl leucine (iDiLeu). These labels contain an amine reactive group, triazine ester, are cost effective because of their synthetic simplicity, and have increased throughput compared with previous LC-MS quantification methods by allowing construction of a four-point standard curve in one run. iDiLeu-labeled peptides show remarkably similar retention time shifts, slightly lower energy thresholds for higher-energy collisional dissociation (HCD) fragmentation, and high quantification accuracy for trypsin-digested protein samples (median errors <15%). By spiking in an iDiLeu-labeled neuropeptide, allatostatin, into mouse urine matrix, two quantification methods are validated. The first uses one labeled peptide as an internal standard to normalize labeled peptide peak areas across runs (<19% error), whereas the second enables standard curve creation and analyte quantification in one run (<8% error).
Greer T, Lietz CB, Xiang F, Li L. J Am Soc Mass Spectrom. 2015 Jan;26(1):107-19. doi: 10.1007/s13361-014-1012-y. Epub 2014 Nov 7.
Herein, we describe the development and application of a set of novel N,N-dimethyl leucine (DiLeu) 4-plex isobaric tandem mass (MS(2)) tagging reagents with high quantitation efficacy and greatly reduced cost for neuropeptide and protein analysis. DiLeu reagents serve as attractive alternatives for isobaric tags for relative and absolute quantitation (iTRAQ) and tandem mass tags (TMTs) due to their synthetic simplicity, labeling efficiency, and improved fragmentation efficiency. DiLeu reagent resembles the general structure of a tandem mass tag in that it contains an amine reactive group (triazine ester) targeting the N-terminus and epsilon-amino group of the lysine side chain of a peptide, a balance group, and a reporter group. A mass shift of 145.1 Da is observed for each incorporated label. Intense a(1) reporter ions at m/z 115.1, 116.1, 117.1, and 118.1 are observed for all pooled samples upon MS(2). All labeling reagents are readily synthesized from commercially available chemicals with greatly reduced cost. Labels 117 and 118 can be synthesized in one step and labels 115 and 116 can be synthesized in two steps. Both DiLeu and iTRAQ reagents show comparable protein sequence coverage (approximately 43%) and quantitation accuracy (<15%) for tryptically digested protein samples. Furthermore, enhanced fragmentation of DiLeu labeling reagents offers greater confidence in protein identification and neuropeptide sequencing from complex neuroendocrine tissue extracts from a marine model organism, Callinectes sapidus.Xiang F, Ye H, Chen R, Fu Q, Li L., Anal Chem. 2010 Apr 1;82(7):2817-25. doi: 10.1021/ac902778d.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| SIL-001-d0 | iDiLeu-d0 | 10 mg | $317 |
| SIL-001-d12 | iDiLeu-d12 | 10 mg | $1238 |
| SIL-001-d3 | iDiLeu-d3 | 10 mg | $444 |
| SIL-001-d6 | iDiLeu-d6 | 10 mg | $571 |
| SIL-001-d9 | iDiLeu-d9 | 10 mg | $952 |
Social Network Confirmation