Abstract: Clinical evidence highlights a relationship between the blood and the bone, but the underlying mechanism linking these two tissues is not fully elucidated. Here, we used β-thalassemia as a model of congenital anemia with bone and bone marrow (BM) niche defects. We demonstrate that fibroblast growth factor 23 (FGF23) is increased in patients and mice with β-thalassemia because erythropoietin induces FGF23 overproduction in bone and BM erythroid cells via ERK1/2 and STAT5 pathways. We show that in vivo inhibition of FGF23 signaling by carboxyl-terminal FGF23 peptide is a safe and efficacious therapeutic strategy to rescue bone mineralization and deposition in mice with β-thalassemia, normalizing the expression of niche factors and restoring hematopoietic stem cell (HSC) function. FGF23 may thus represent a molecular link connecting anemia, bone, and the HSC niche. This study provides a translational approach to targeting bone defects and rescuing HSC niche interactions, with potential clinical relevance for improving HSC transplantation and gene therapy for hematopoietic disorders.
Aprile A, Raggi L, Bolamperti S, et al. Inhibition of FGF23 is a therapeutic strategy to target hematopoietic stem cell niche defects in β-thalassemia. Sci Transl Med. 2023;15(698):eabq3679.
Abstract: Fibroblast growth factor 23 (FGF23) is a bone-derived hormone that binds to binary FGFR/alpha-Klotho receptor complexes in the kidney tubules to inhibit phosphate reabsorption and 1,25(OH)2D production. Excess FGF23 causes X-linked hypophosphatemia (XLH) and tumor induced osteomalacia (TIO). Recently, Burosumab, an FGF23 blocking antibody, was approved for treating these hypophosphatemic disorders.The authors used Phoenix’s cFGF-23 (Human) peptide (Cat # 073-62) to demonstrate results.Xiao Z, Liu J, Liu SH, et al. Small Molecule FGF23 Inhibitors Increase Serum Phosphate and Improve Skeletal Abnormalities in Hyp Mice. Pharmacology and Toxicology; 2020.
Abstract: Hypoferremia results as an acute phase response to infection and inflammation aiming to reduce iron availability to pathogens. Activation of toll-like receptors (TLRs), the key sensors of the innate immune system, induces hypoferremia mainly through the rise of the iron hormone hepcidin. Conversely, stimulation of erythropoiesis suppresses hepcidin expression via induction of the erythropoietin-responsive hormone erythroferrone. Iron deficiency stimulates transcription of the osteocyte-secreted protein FGF23. Here we hypothesized that induction of FGF23 in response to TLR4 activation is a potent contributor to hypoferremia and, thus, impairment of its activity may alleviate hypoferremia induced by lipopolysaccharide (LPS), a TLR 4 agonist. We used the C-terminal tail of FGF23 to impair endogenous full-length FGF23 signaling in wild-type mice, and investigated its impact on hypoferremia. Our data show that FGF23 is induced as early as pro-inflammatory cytokines in response to LPS, followed by upregulation of hepcidin and downregulation of erythropoietin (Epo) expression in addition to decreased serum iron and transferrin saturation. Further, LPS-induced hepatic and circulating hepcidin were significantly reduced by FGF23 signaling disruption. Accordingly, iron sequestration in liver and spleen caused by TLR4 activation was completely abrogated by FGF23 signaling inhibition, resulting in alleviation of serum iron and transferrin saturation deficit. Taken together, our studies highlight for the first time that inhibition of FGF23 signaling alleviates LPS-induced acute hypoferremia.
Rafiou Agoro, Min Young Park, Carole Le Henaff, et al. C-FGF23 peptide alleviates hypoferremia during acute inflammation. haematol. 2020;106(2):391-403
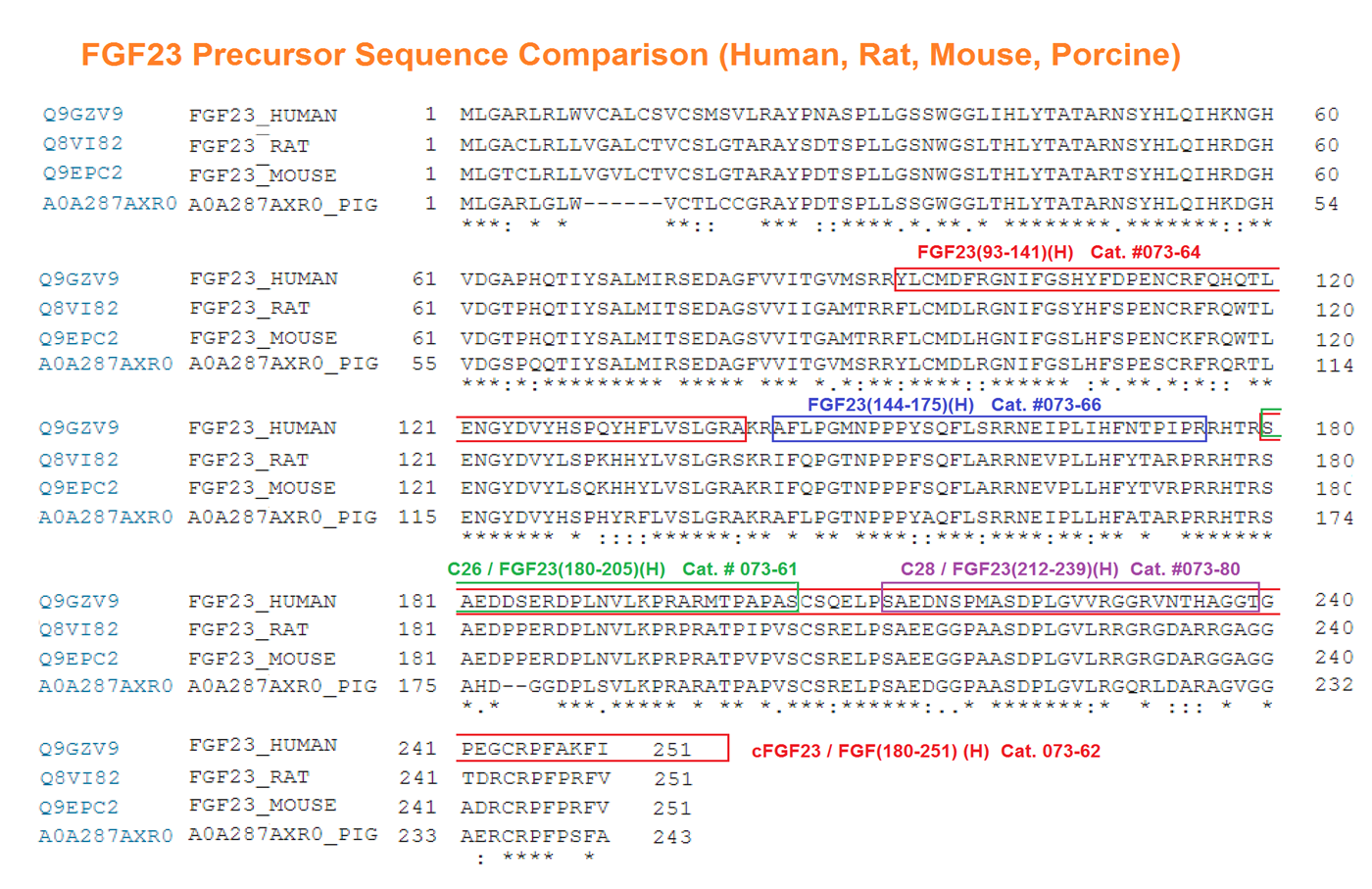
Abstract: FGF23 interacts with a FGFR/KL-receptor complex to propagate cellular signaling, where its C-terminal C26 peptide is critical for engaging the co-receptor KL. We identify a distinct peptide sequence C28 residing in the FGF23 C terminus that regulates its interaction with KL. C28 can independently function as an FGF23 antagonist, and we report an optimized peptide antagonist of much enhanced potency. FGF23 can use either of the two C-terminal sites to exert biological effects, as shown by in vitro and in vivo studies. The loss of both KL-interaction sites inactivates the protein. We conclude that the C terminus of FGF23 is a bidentate ligand possessing two independent KL-interaction sites. The identification of this second KL-association site provides an additional perspective in the molecular basis of FGF23-receptor signaling and raises questions pertaining to its structural mechanism of action and the potential for biased biological signaling.
Agrawal A, Ni P, Agoro R, White KE, DiMarchi RD. Identification of a second Klotho interaction site in the C terminus of FGF23. Cell Reports. 2021;34(4):108665
Abstract: Fibroblast Growth Factor 23 (FGF23) is a key hormone for the regulation of phosphate homeostasis. Over the past decades, FGF23 was the subject of intense research in the fields of nephrology and the cardiology. It presents a remarkable correlation with well-established biomarkers of cardiovascular disorders in both chronic kidney disease (CKD) and heart failure (HF) patients. The interest of FGF23 lies in its early-onset in the primary course of CKD as well as in the incremental prognosis information it conveys in both CKD and HF. Different types of assays of FGF-23 testing exist, those targeting the intact form (iFGF23), the other one detecting terminal fragments (cFGF23). The issue is still pending which assay suits best for clinical use. Recently, the implementation of this biomarker on multianalyzer platforms, on which other markers of phospho-calcic balance are set up, allows a rapid turn-around-time and a potential financial gain. However, despite the good analytical performances of the automated methods, there is a poor harmonization between assays. The introduction of an international certified reference material should standardize the measurement and improve the harmonization of results from different laboratories. A deeper understanding of physio-pathological mechanisms and processing of FGF-23 should reinforce its clinical indications and might also identify new therapeutic targets for the treatment of CKD and HF.
Fauconnier C, Roy T, Gillerot G, Roy C, Pouleur AC, Gruson D. FGF23: Clinical usefulness and analytical evolution. Clinical Biochemistry. 2019;66:1-12.
Abstract: Fibroblast growth factor 23 (FGF23) production is upregulated by iron deficiency and hypoxia. However, the influence of acute blood loss, and the resulting increases in circulating erythropoietin, on FGF23 production is unknown. Using wild-type C57BL/6 mice, we show that acute loss of 10% total blood volume leads to an increase in plasma C-terminal FGF23 (cFGF23) levels within 6 h, while plasma levels of intact FGF23, phosphate, calcium, parathyroid hormone, iron, and ferritin remain similar to control mice without acute blood loss. Volume resuscitation with PBS did not significantly alter these findings. The increase in plasma cFGF23 levels in bled animals was accompanied by increased plasma erythropoietin levels at 6 h. Administration of erythropoietin led to an acute increase in plasma cFGF23 levels similar to that observed in acute blood loss. Fgf23 mRNA expression was increased 20-fold in bone marrow, but not in bone, of bled vs. control mice, suggesting bone marrow as a key source of elevated plasma FGF23 levels following acute blood loss. To extend these findings to humans, we measured plasma cFGF23 levels in 131 critically ill patients admitted to the intensive care unit. In univariate and multivariate models, we found a positive association between number of red blood cell transfusions, an indirect indicator of acute blood loss, and plasma cFGF23 levels. We conclude that FGF23 production is rapidly increased after acute blood loss and that erythropoietin may be the mediator of this increase. Thus erythropoietin may represent a novel physiological regulator of FGF23 production.
Rabadi S, Udo I, Leaf DE, Waikar SS, Christov M. Acute blood loss stimulates fibroblast growth factor 23 production. American Journal of Physiology-Renal Physiology. 2018;314(1):F132-F139.
Abstract: Fibroblast growth factor-23 (FGF-23) interacts with a binary receptor complex composed of alpha-Klotho (alpha-KL) and FGF receptors (FGFRs) to regulate phosphate and vitamin D metabolism in the kidney. Excess FGF-23 production, which causes hypophosphatemia, is genetically inherited or occurs with chronic kidney disease. Among other symptoms, hypophosphatemia causes vitamin D deficiency and the bone-softening disorder rickets.
The authors used Phoenix’s cFGF-23 (Human) peptide (Cat # 073-62) to demonstrate results.
Xiao Z, Riccardi D, Velazquez HA, et al. A computationally identified compound antagonizes excess FGF-23 signaling in renal tubules and a mouse model of hypophosphatemia. Sci Signal. 2016;9(455).
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| MB-073-62 | cFGF / FGF23 (180-251) (Human)- MagBead (Magnetic Bead Linked Antibody) | 1 ml | $796 |
| 073-61 | C26 / FGF23-R1 / FGF23 (180-205) (Human) | 100 µg | $253 |
| 073-80 | C28 / FGF23-R2 / FGF23 (212-239 ) (Human) | 100 µg | $328 |
| 073-86 | C28 optimized peptide 15 Dimer (Human) | 100 µg | $397 |
| 073-84 | C28 optimized peptide 15 Monomer (Human) | 100 µg | $244 |
| 073-82 | C28 optimized peptide 6 (Human) | 100 µg | $222 |
| 073-66 | FGF23 (144-175 ) (Human) | 100 µg | $328 |
| EK-073-62 | FGF23 (180-251) / cFGF (Human)- EIA Kit | 96 wells | $610 |
| 073-88 | FGF23 (188-199) optimized peptide 10 (Human) | 100 µg | $244 |
| 073-64 | FGF23 (93-141) (Human) | 100 µg | $328 |
Social Network Confirmation