Ca2+ plays a pivotal role in both excitation-contraction coupling (ECC) and activation of Ca2+-dependent signaling pathways. One of the remaining questions in cardiac biology is how Ca2+-dependent signaling pathways are regulated under conditions of continual Ca2+ transients that mediate cardiac contraction during each heartbeat. Ca2+-calmodulin-dependent protein kinase II (CaMKII) activation and its ability to regulate histone deacetylase 5 (HDAC5) nuclear shuttling represent a critical Ca2+-dependent signaling circuit for controlling cardiac hypertrophy and heart failure, yet the mechanism of activation by Ca2+ is not known. In this issue of the JCI, Wu et al. convincingly demonstrate that the inositol 1,4,5-trisphosphate receptor (InsP3R) is involved in local control of Ca2+ for activating CaMKII in the nuclear envelope of adult ventricular cardiac myocytes (see the related article beginning on page 675). The overall paradigm that is demonstrated is the best example of a molecular mechanism whereby signaling is directly regulated by a local Ca2+ pool that is disparate or geometrically insensitive to cytosolic Ca2+ underlying each contractile cycle.
Molkentin JD. Dichotomy of Ca2+ in the heart: contraction versus intracellular signaling. J Clin Invest. 2006;116(3):623-6.
Neurotrophic growth factors, including nerve growth factor (NGF) and glial-derived neurotrophic factor (GDNF), have well-established roles in promoting the innervation of target tissues, yet little is known about how the temporal and organ-specific expression of these factors is regulated. A new study reveals that NGF is a direct target of the well-characterized peptide factor endothelin-1 (ET-1), and that ET-1–induced NGF expression is required for sympathetic innervation of the developing heart. These results, and recent studies implicating GDNF and ET-3 in the patterning of the enteric nervous system, suggest that specific pairing of endothelins and neurotrophic factors may be used in distinct target organs to coordinate neuronal migration, differentiation, and survival.
Hempstead BL. Sculpting organ innervation. Journal of Clinical Investigation. 2004;113(6):811-813. doi:10.1172/JCI200421309.
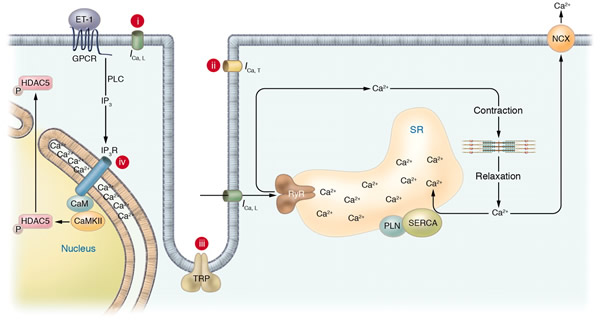 |
Schematic of potential Ca2+ sources that might be specialized to regulate reactive signaling pathways in cardiac myocytes. (i) Some L-type Ca2+ channels (ICa,L) are not associated with the junctional complex and hence could be involved in providing a local Ca2+ signal in specific membrane-associated compartments. (ii) T-type Ca2+ channels (ICa,T) are reexpressed in hypertrophic states where they could provide Ca2+ in specific microenvironments associated with the sarcolemma to affect reactive signaling pathways. (iii) Capacitative or store-operated Ca2+ entry through transient receptor potential (TRP) channels, alone or in conjunction with (iv) InsP3R-mediated release of Ca2+ from the ER/nuclear envelope, could also provide a highly localized Ca2+ pool for controlling reactive signaling pathways in cardiac myocytes. Signaling from G protein–coupled receptors (GPCRs) activates PLC and generates InsP3, causing a perinuclear Ca2+ signal through the InsP3R, resulting in CamKII activation and HDAC5 nuclear export, as proposed by Wu et al. (2). IP3, InsP3; IP3R, InsP3R; PLN, phospholamban; NCX, Na+/Ca2+ exchanger; RyR, ryanodine receptor; CaM, calmodulin; SERCA, SR/ER Ca2+-ATPase; P, phosphate.
Molkentin, J. D. J. Clin. Invest. 2006;116:623-626 |
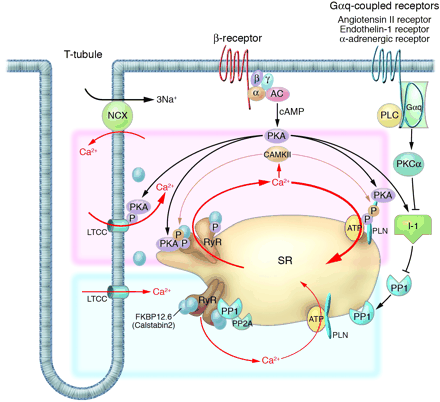 |
Intracellular Ca2+ cycling and associated signaling pathway in cardiomyocytes. On a beat-by-beat basis, a calcium transient is evoked by the initial influx of a small amount of Ca2+ through the LTCC and the subsequent large-scale Ca2+ release from the SR through the RyR. During diastole, cytosolic Ca2+ is taken up into the SR by the PLN-regulated SERCA2a pump. ß receptor–mediated PKA stimulation regulates this Ca2+ cycling by phosphorylating LTCC, RyR, and PLN. In normal hearts, sympathetic stimulation activates ß1-adrenergic receptor, which in turn stimulates the production of cAMP by adenylyl cyclase and thereby activates PKA. PKA phosphorylates PLN and RyR, both of which contribute to an increased intracellular Ca2+ transient and enhanced cellular contractility (pink zone signal). PP1 and PP2A regulate the dephosphorylation process of these Ca2+ regulatory proteins (RyR, PLN, LTCC) (blue zone signaling). Activation of the Gq-coupled receptors (angiotensin II receptor, endothelin 1 receptor, or -adrenergic receptor) activates PLC, which in turn activates PKC-. The PKC- phosphorylates I-1, augmenting the activity of PP1 and causing hypophosphorylation of PLB. The PLB hypophosphorylation inhibits SERCA2a activity, thereby decreasing SR Ca2+ uptake. The increased Ca2+ level in the cytosol activates CAMKII, which affects the functions of RyR and PLN. Activation or deactivation of these molecules at a node in the signaling cascade affects beat-by-beat Ca2+ cycling, and such maneuvers have recently been highlighted as potential new therapeutic strategies against HF. , G protein subunit ; ß, G protein subunit ß; , G protein subunit ; AC, adenylyl cyclase; PLC, phospholipase C. |
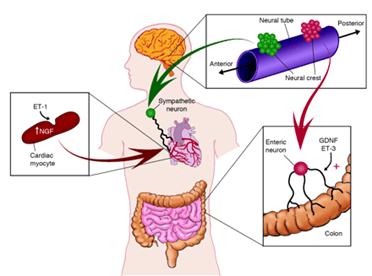 |
Distinctive pairing of endothelins and neurotrophic factors promotes target innervation during development. Although the sequential action of ET-1 to induce myocyte production of NGF is suggested to promote sympathetic innervation of the heart, more complex interactions of ET-3 and GDNF occur in the patterning of the enteric nervous system.
Barbara L. Hempstead J. Clin. Invest. 113:811-813 (2004) |
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 023-01 | Endothelin 1 (ET-1) (Human, Rat, Mouse, Porcine, Bovine, Canine, Rabbit, Monkey) | 100 µg | $108 |
| 023-38 | Endothelin B Receptor Antagonist / BQ-788 | 200 µg | $96 |
| EK-023-01 | Endothelin 1 (ET-1) (Human, Rat, Mouse, Porcine, Bovine, Canine, Rabbit, Monkey) - EIA Kit | 96 wells | $570 |
| RK-023-01 | Endothelin 1 (ET-1) (Human, Rat, Mouse, Porcine, Bovine, Canine, Rabbit, Monkey) - RIA Kit | 125 tubes | $880 |
| 023-10 | Endothelin 1 (ET-1), big (Human) | 100 µg | $146 |
| EK-023-01CE | Endothelin 1 (ET-1) (Human, Rat, Mouse, Porcine, Bovine, Canine, Rabbit, Monkey) - EIA Kit, CE Mark Certified | 96 wells | $595 |
| 023-07 | [Ala11, Ala15]-Endothelin 1 (6-21) / 2Ala-ET-1 (6-21) (Human, Rat, Mouse, Porcine, Bovine, Canine, Rabbit, Monkey) | 200 µg | $55 |
| 023-53 | [Suc0, Glu9, Ala11, Ala15]-Endothelin 1 (8-21) / IRL-1620 | 200 µg | $48 |
| 023-28 | prepro-Endothelin 1 (ET-1) (108-138) (Porcine) | 100 µg | $172 |
| 023-18 | prepro-Endothelin 1 (ET-1) (109-125) amide (Human) | 100 µg | $299 |
Social Network Confirmation