Catalog # |
Size |
Price |
|
|---|---|---|---|
| EK-070-94 | 96 wells | $576 |
| View/Download (PDF) - for reference only | |||||||||||||||||||||
 To reduce background noise for this kit, do not add EDTA to your samples. Heparin can be used as an alternative to EDTA for this kit.
| Recommended | ||||||||||||||||||||
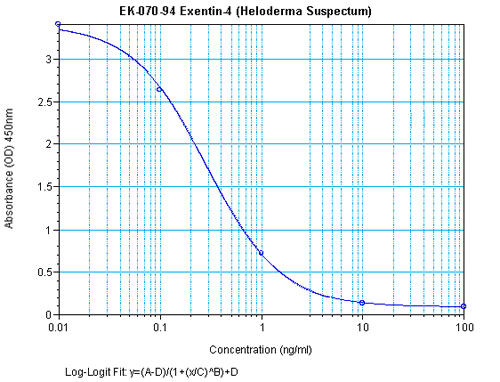
 Data may differ slightly based on lot. | 0.08 ng/ml | ||||||||||||||||||||
 Data may differ slightly based on lot. | 0.08 - 0.86 ng/ml | ||||||||||||||||||||
| |||||||||||||||||||||
|
| <10% | ||||||||||||||||||||
|
| <15% | ||||||||||||||||||||
|
|
Store the kit at 4°C | ||||||||||||||||||||
|
| To reduce background noise for this kit, do not add EDTA to your samples.Heparin can be used as an alternative to EDTA for this kit. | ||||||||||||||||||||
C-terminal site-specific PEGylated Exendin-4 analog: A long-acting glucagon like Peptide-1 receptor agonist, on glycemic control and beta cell function in diabetic db/db mice.
Tang D, Tian H, Wu J, et al. J Pharmacol Sci. 2018;138(1):23-30.
GLP-1 receptor agonists stimulate ANGPTL8 production through the PI3K/Akt pathway in a GLP-1 receptor-dependent manner.
Liu J, Yang K, Xiao W, et al. Peptides. 2018;106:83-90.
The use of low molecular weight protamine to enhance oral absorption of exenatide.
Zhang L, Shi Y, Song Y, et al. Int J Pharm. 2018;547(1-2):265-273.
Replacement of the C-terminal Trp-cage of exendin-4 with a fatty acid improves therapeutic utility.
Lee JG, Ryu JH, Kim SM, et al. Biochem Pharmacol. 2018;151:59-68.
Sustained-release study on Exenatide loaded into mesoporous silica nanoparticles: in vitro characterization and in vivo evaluation.
Chen C, Zheng H, Xu J, Shi X, Li F, Wang X. Daru. 2017;25(1):20.
In vitro and in vivo characterization of a novel long-acting GLP-1 receptor agonist, exendin-4–Fc fusion protein
Lian Lu, Xiaoqing Su, Yantai Wang et al., RSC Adv., 2017,7, 54178-54187
An approach for half-life extension and activity preservation of an anti-diabetic peptide drug based on genetic fusion with an albumin-binding aptide.
Kim D, Jeon H, Ahn S, Choi WI, Kim S, Jon S. J Control Release. 2017;256:114-120.
Population pharmacodynamic modeling of exenatide after 2-week treatment in STZ/NA diabetic rats.
Chen T, Kagan L, Mager DE. J Pharm Sci. 2013;102(10):3844-51.
Combination therapy via oral co-administration of insulin- and exendin-4-loaded nanoparticles to treat type 2 diabetic rats undergoing OGTT.
Chuang EY, Nguyen GT, Su FY, et al. Biomaterials. 2013;34(32):7994-8001.
Exenatide-loaded PLGA microspheres with improved glycemic control: in vitro bioactivity and in vivo pharmacokinetic profiles after subcutaneous administration to SD rats.
Xuan J, Lin Y, Huang J, et al. Peptides. 2013;46:172-9.
Oral delivery of an anti-diabetic peptide drug via conjugation and complexation with low molecular weight chitosan.
Ahn S, Lee IH, Lee E, Kim H, Kim YC, Jon S. J Control Release. 2013;170(2):226-32.
Delivery of two-step transcription amplification exendin-4 plasmid system with arginine-grafted bioreducible polymer in type 2 diabetes animal model.
Kim PH, Lee M, Kim SW. J Control Release. 2012;162(1):9-18.
Sustained exendin-4 secretion through gene therapy targeting salivary glands in two different rodent models of obesity/type 2 diabetes.
Di pasquale G, Dicembrini I, Raimondi L, et al. PLoS ONE. 2012;7(7):e40074.
Site-specific PEGylation of exenatide analogues markedly improved their glucoregulatory activity.
Gong N, Ma A-N, Zhang L-J, et al. British Journal of Pharmacology. 2011;163(2):399-412.
Pharmacokinetic and Pharmacodynamic Modeling of Exendin-4 in Type 2 Diabetic Goto-Kakizaki Rats.
Gao W, Jusko WJ. The Journal of Pharmacology and Experimental Therapeutics. 2011;336(3):881-890.
Biochemical, pharmaceutical and therapeutic properties of long-acting lithocholic acid derivatized exendin-4 analogs.
Chae SY, Jin CH, Shin JH, et al. J Control Release. 2010;142(2):206-13.
Importance of intermolecular interaction on the improvement of intestinal therapeutic peptide/protein absorption using cell-penetrating peptides.
Kamei N, Morishita M, Takayama K. J Control Release. 2009;136(3):179-86.
Gene therapy for diabetes: metabolic effects of helper-dependent adenoviral exendin 4 expression in a diet-induced obesity mouse model.
Samson SL, Gonzalez EV, Yechoor V, Bajaj M, Oka K, Chan L. Mol Ther. 2008;16(11):1805-12.
Social Network Confirmation