Bidirectional signaling by cell adhesion molecules is thought to mediate synapse formation, but the mechanisms involved remain elusive. We found that the adhesion G protein-coupled receptors latrophilin-2 and latrophilin-3 selectively direct formation of perforant-path and Schaffer-collateral synapses, respectively, to hippocampal CA1-region neurons. Latrophilin-3 binds to two transcellular ligands: fibronectin leucine-rich repeat transmembrane proteins (FLRTs) and teneurins. In transgenic mice in vivo, both binding activities were required for input-specific synapse formation, which suggests that coincident binding of both ligands is necessary for synapse formation. In cultured neurons in vitro, teneurin or FLRT alone did not induce excitatory synapse formation, whereas together they potently did so. Thus, postsynaptic latrophilins promote excitatory synapse formation by simultaneous binding of two unrelated presynaptic ligands, which is required for formation of synaptic inputs at specific dendritic localizations.Sando R, Jiang X, Südhof TC. Latrophilin GPCRs direct synapse specificity by coincident binding of FLRTs and teneurins. Science. 2019;363(6429)
The teneurins are a family of four transmembrane proteins essential to intercellular adhesion processes, and are required for the development and maintenance of tissues. The Adhesion G protein-coupled receptor (GPCR) subclass latrophilins (ADGRL), or simply the latrophilins (LPHN), are putative receptors of the teneurins and act, in part, to mediate intercellular adhesion via binding with the teneurin extracellular region. At the distal tip of the extracellular region of each teneurin lies a peptide sequence termed the teneurin C-terminal associated peptide (TCAP). TCAP-1, associated with teneurin-1, is itself bioactive, suggesting that TCAP is a critical functional region of teneurin. However, the role of TCAP-1 has not been established with respect to its ability to interact with LPHN to induce downstream effects. To establish that TCAP-1 binds to LPHN1, a FLAG-tagged hormone binding domain (HBD) of LPHN1 and a GFP-tagged TCAP-1 peptidewere co-expressed in HEK293 cells. Both immunoreactive epitopes were co-localized as a single band after immunoprecipitation, indicating an association between the two proteins. Moreover, fluorescent co-labeling occurred at the plasma membrane of LPHN1 over-expressing cells when treated with a FITC-tagged TCAP-1 variant. Expression of LPHN1 and treatment with TCAP-1 modulated the actin-based cytoskeleton in these cells in a manner consistent with previously reported actions of TCAP-1 and affected the overall morphology and aggregation of the cells. This study indicates that TCAP-1 may associate directly with LPHN1 and could play a role in the modulation of cytoskeletal organization and intercellular adhesion and aggregation via this interaction.Husi? M, Barsyte-lovejoy D, Lovejoy DA. Teneurin C-Terminal Associated Peptide (TCAP)-1 and Latrophilin Interaction in HEK293 Cells: Evidence for Modulation of Intercellular Adhesion. Front Endocrinol (Lausanne). 2019;10:22.
Teneurin C-terminal associated peptide (TCAP) is an ancient paracrine signalling agent that evolved via lateral gene transfer from prokaryotes into an early metazoan ancestor. Although it bears structural similarity to corticotrophin-releasing hormone (CRH), it inhibits the in vivo actions of CRH. The TCAPs are highly expressed in neurones, where they induce rapid cytoskeletal rearrangement and are neuroprotective. Because these processes are highly energy-dependent, this suggests that TCAP has the potential to regulate glucose uptake because glucose is the primary energy substrate in brain, and neurones require a steady supply to meet the high metabolic demands of neuronal communication. Therefore, the objective of the present study was to assess the effect of TCAP-mediated glucose uptake in the brain and in neuronal cell models. TCAP-mediated 18 F-deoxyglucose (FDG) uptake into brain tissue was assessed in male wild-type Wistar rats by functional positron emission tomography. TCAP-1 increased FDG uptake by over 40% into cortical regions of the brain, demonstrating that TCAP-1 can significantly enhance glucose supply. Importantly, a single nanomolar injection of TCAP-1 increased brain glucose after 3 days and decreased blood glucose after 1 week. This is corroborated by a decreased serum concentration of insulin and an increased serum concentration of glucagon. In immortalised hypothalamic neurones, TCAP-1 increased ATP production and enhanced glucose uptake by increasing glucose transporter recruitment to the plasma membrane likely via AKT and mitogen-activated protein kinase/ERK phosphorylation events. Taken together, these data demonstrate that TCAP-1 increases glucose metabolism in neurones, and may represent a peptide signalling agent that regulated glucose uptake before insulin and related peptides.Hogg DW, Chen Y, D'aquila AL, et al. A novel role of the corticotrophin-releasing hormone regulating peptide, teneurin C-terminal associated peptide 1, on glucose uptake into the brain. J Neuroendocrinol. 2018;30(4):e12579.
Teneurins (TENs) are cell-surface adhesion proteins with critical roles in tissue development and axon guidance. Here, we report the 3.1-Å cryoelectron microscopy structure of the human TEN2 extracellular region (ECR), revealing a striking similarity to bacterial Tc-toxins. The ECR includes a large β barrel that partially encapsulates a C-terminal domain, which emerges to the solvent through an opening in the mid-barrel region. An immunoglobulin (Ig)-like domain seals the bottom of the barrel while a β propeller is attached in a perpendicular orientation. We further show that an alternatively spliced region within the β propeller acts as a switch to regulate trans-cellular adhesion of TEN2 to latrophilin (LPHN), a transmembrane receptor known to mediate critical functions in the central nervous system. One splice variant activates trans-cellular signaling in a LPHN-dependent manner, whereas the other induces inhibitory postsynaptic differentiation. These results highlight the unusual structural organization of TENs giving rise to their multifarious functions.Li J, Shalev-benami M, Sando R, et al. Structural Basis for Teneurin Function in Circuit-Wiring: A Toxin Motif at the Synapse. Cell. 2018;173(3):735-748.e15.
Alzheimer's disease is a debilitating age-related disorder characterized by distinct pathological hallmarks, such as progressive memory loss and cognitive impairment. During the last few years, several cellular signaling pathways have been associated with the pathogenesis of Alzheimer's disease, such as Notch, mTOR and Wnt. However, the potential factors that modulate these pathways and novel molecular mechanisms that might account for the pathogenesis of Alzheimer's disease or for therapy against this disease are still matters of intense research. Teneurins are members of a unique protein system that has recently been proposed as a novel and highly conserved regulatory signaling system in the vertebrate brain, so far related with neurite outgrowth and neuronal matching. The similitude in structure and function of teneurins with other cellular signaling pathways, suggests that they may play a critical role in Alzheimer's disease, either through the modulation of transcription factors due to the nuclear translocation of the teneurins intracellular domain, or through the activity of the corticotrophin releasing factor (CRF)-like peptide sequence, called teneurin C-terminal associated peptide. Moreover, the presence of Ca(2+)-binding motifs within teneurins structure and the Zic2-mediated Wnt/β-catenin signaling modulation, allows hypothesize a potential crosslink between teneurins and the Wnt signaling pathway, particularly. Herein, we aim to highlight the main characteristics of teneurins and propose, based on current knowledge of this family of proteins, an interesting review of their potential involvement in Alzheimer's disease.Bastías-candia S, Braidy N, Zolezzi JM, Inestrosa NC. Teneurins and Alzheimer's disease: a suggestive role for a unique family of proteins. Med Hypotheses. 2015;84(4):402-7.
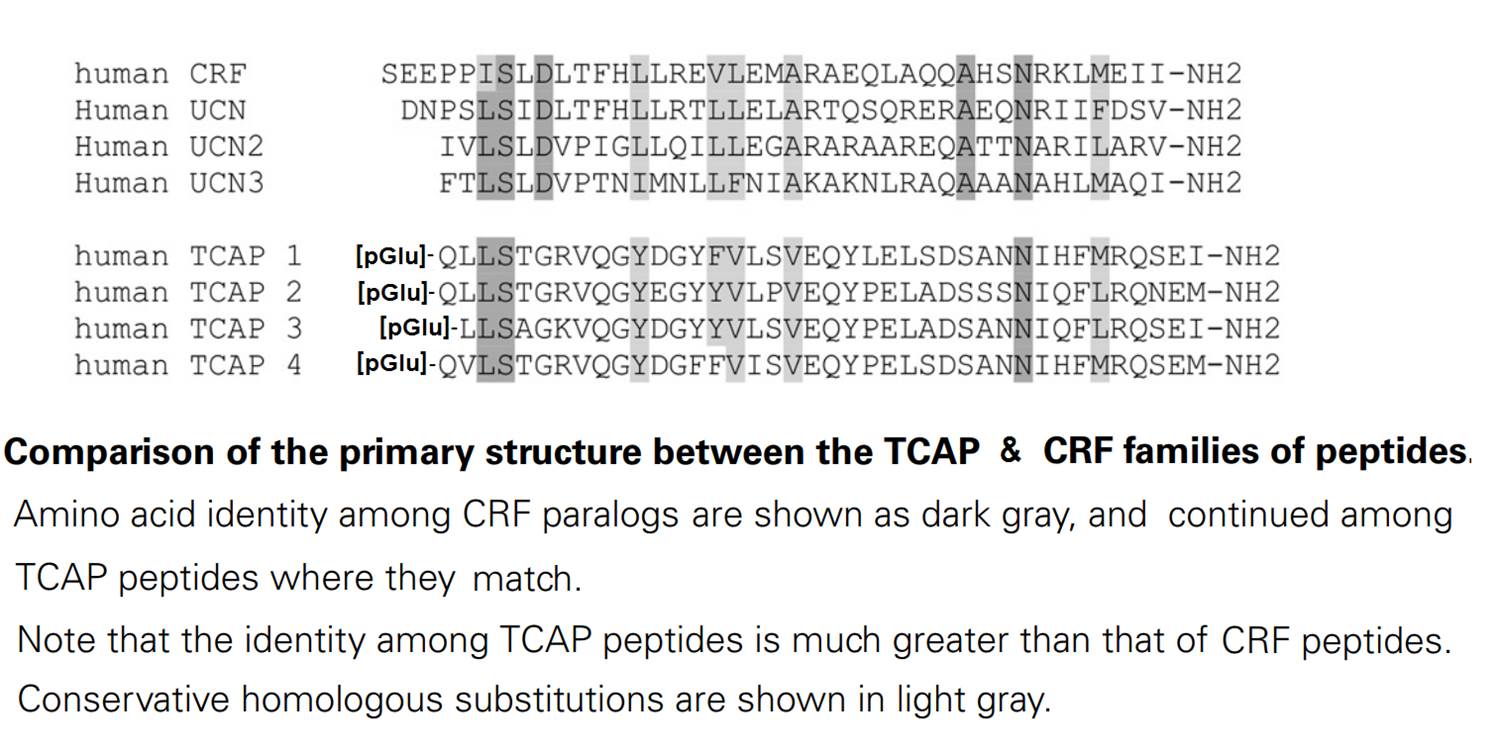
Neuropeptides that evolved early in metazoan evolution may possess much larger networks of paralogous genes than later evolving peptides due to the increased exposure to gene and genomic duplication events. The corticotropin-releasing factor family of peptides, which also include invertebrate CRF-like peptides, are a candidate group that appear to have an early origin. We have attempted to find additional paralogous genes to the CRF family by doing a low-stringency screen of a rainbow trout hypothalamic cDNA library using a hamster urocortin probe. A clone was identified that represented the rainbow trout ortholog of teneurin-3. The C-terminal region of the last exon teneurin transmembrane protein gene possesses a neuropeptide-like sequence with a primary structure similarity to the corticotropin-releasing factor family of peptides. We have called this sequence teneurin C-terminal associated peptide (TCAP). The predicted peptide is 40 residues long and possesses an expected pyroglutamyl residue in the first position and an amidated carboxy terminus. A synthetic version of the rainbow trout (rt) TCAP-3 is potent at increasing the concentration of cAMP and stimulating proliferation in a neuronal cell line. The synthetic peptide can also either increase or decrease the expression of the teneurin-1 gene, depending upon its concentration. The teneurin/TCAP system may represent a novel and highly conserved regulatory signalling system in the vertebrate brain.Qian X, Barsyte-lovejoy D, Wang L, et al. Cloning and characterization of teneurin C-terminus associated peptide (TCAP)-3 from the hypothalamus of an adult rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol. 2004;137(2):205-16.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 020-16 | TCAP-1 / Teneurin C-terminal Associated Peptide-1 (Human) | 100 µg | $300 |
| B-020-16 | [Biotinyl-Gln1]-TCAP-1 / Teneurin C-terminal Associated Peptide-1 (Human) | 20 µg | $317 |
| 020-18 | TCAP-2 / Teneurin C-terminal Associated Peptide-2 (Human) | 100 µg | $300 |
| B-020-18 | [Biotinyl-Gln1]-TCAP-2 / Teneurin C-terminal Associated Peptide-2 (Human) | 20 µg | $317 |
| 020-20 | TCAP-3 / Teneurin C-terminal Associated Peptide-3 (Human) | 100 µg | $300 |
| B-020-20 | [Biotinyl-Gln1]-TCAP-3 / Teneurin C-terminal Associated Peptide-3 (Human) | 20 µg | $317 |
| 020-22 | TCAP-4 / Teneurin C-terminal Associated Peptide-4 (Human) | 100 µg | $300 |
| B-020-22 | [Biotinyl-Gln1]-TCAP-4 / Teneurin C-terminal Associated Peptide-4 (Human) | 20 µg | $317 |
Social Network Confirmation