

Objective: The insulin/IGF superfamily is conserved across vertebrates and invertebrates. Our team has identified five viruses containing genes encoding viral insulin/IGF-1 like peptides (VILPs) closely resembling human insulin and IGF-1. This study aims to characterize the impact of Mandarin fish ranavirus (MFRV) and Lymphocystis disease virus-Sa (LCDV-Sa) VILPs on the insulin/IGF system for the first time.
Methods: We chemically synthesized single chain (sc, IGF-1 like) and double chain (dc, insulin like) forms of MFRV and LCDV-Sa VILPs. Using cell lines overexpressing either human insulin receptor isoform A (IR-A), isoform B (IR-B) or IGF-1 receptor (IGF1R), and AML12 murine hepatocytes, we characterized receptor binding, insulin/IGF signaling. We further characterized the VILPs' effects of proliferation and IGF1R and IR gene expression, and compared them to native ligands. Additionally, we performed insulin tolerance test in CB57BL/6 J mice to examine in vivo effects of VILPs on blood glucose levels. Finally, we employed cryo-electron microscopy (cryoEM) to analyze the structure of scMFRV-VILP in complex with the IGF1R ectodomain.
Results: VILPs can bind to human IR and IGF1R, stimulate receptor autophosphorylation and downstream signaling pathways. Notably, scMFRV-VILP exhibited a particularly strong affinity for IGF1R, with a mere 10-fold decrease compared to human IGF-1. At high concentrations, scMFRV-VILP selectively reduced IGF-1 stimulated IGF1R autophosphorylation and Erk phosphorylation (Ras/MAPK pathway), while leaving Akt phosphorylation (PI3K/Akt pathway) unaffected, indicating a potential biased inhibitory function. Prolonged exposure to MFRV-VILP led to a significant decrease in IGF1R gene expression in IGF1R overexpressing cells and AML12 hepatocytes. Furthermore, insulin tolerance test revealed scMFRV-VILP's sustained glucose-lowering effect compared to insulin and IGF-1. Finally, cryo-EM analysis revealed that scMFRV-VILP engages with IGF1R in a manner closely resembling IGF-1 binding, resulting in a highly analogous structure.Conclusions: This study introduces MFRV and LCDV-Sa VILPs as novel members of the insulin/IGF superfamily. Particularly, scMFRV-VILP exhibits a biased inhibitory effect on IGF1R signaling at high concentrations, selectively inhibiting IGF-1 stimulated IGF1R autophosphorylation and Erk phosphorylation, without affecting Akt phosphorylation. In addition, MFRV-VILP specifically regulates IGF-1R gene expression and IGF1R protein levels without affecting IR. CryoEM analysis confirms that scMFRV-VILP' binding to IGF1R is mirroring the interaction pattern observed with IGF-1. These findings offer valuable insights into IGF1R action and inhibition, suggesting potential applications in development of IGF1R specific inhibitors and advancing long-lasting insulins.
Chrudinová M, Kirk NS, Chuard A, et al. A viral insulin-like peptide inhibits IGF-1 receptor phosphorylation and regulates IGF1R gene expression. Molecular Metabolism. 2024;80:101863.
Abstract: The human insulin receptor signalling system plays a critical role in glucose homeostasis. Insulin binding brings about extensive conformational change in the receptor extracellular region that in turn effects trans-activation of the intracellular tyrosine kinase domains and downstream signalling. Of particular therapeutic interest is whether insulin receptor signalling can be replicated by molecules other than insulin. Here, we present single-particle cryoEM structures that show how a 33-mer polypeptide unrelated to insulin can cross-link two sites on the receptor surface and direct the receptor into a signalling-active conformation. The 33-mer polypeptide engages the receptor by two helical binding motifs that are each potentially mimicable by small molecules. The resultant conformation of the receptor is distinct from—but related to—those in extant three-dimensional structures of the insulin-complexed receptor. Our findings thus illuminate unexplored pathways for controlling the signalling of the insulin receptor as well as opportunities for development of insulin mimetics.Kirk NS, Chen Q, Wu YG, et al. Activation of the human insulin receptor by non-insulin-related peptides. Nat Commun. 2022;13(1):5695.
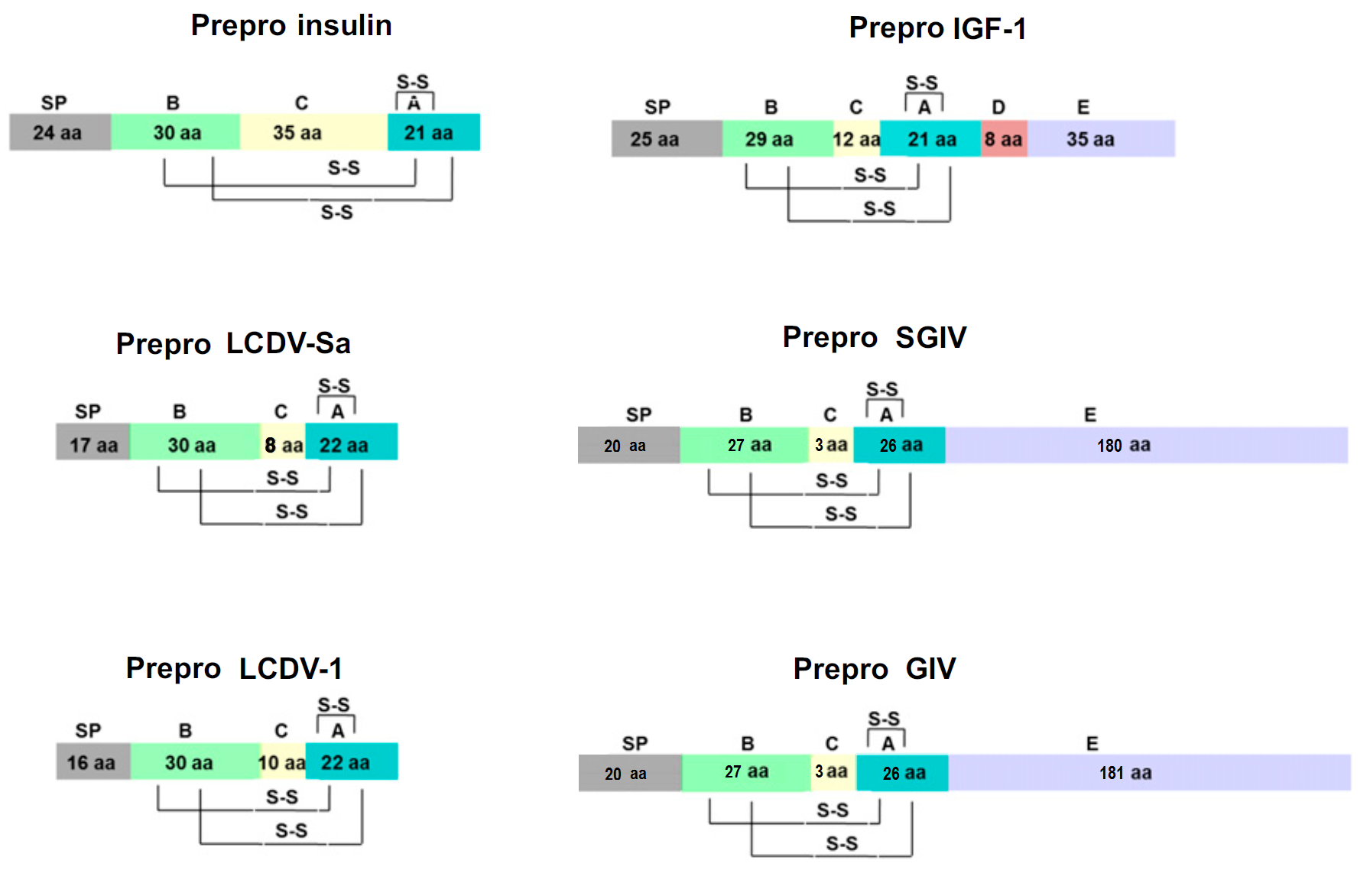
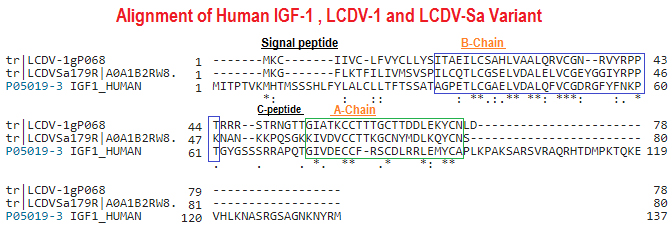
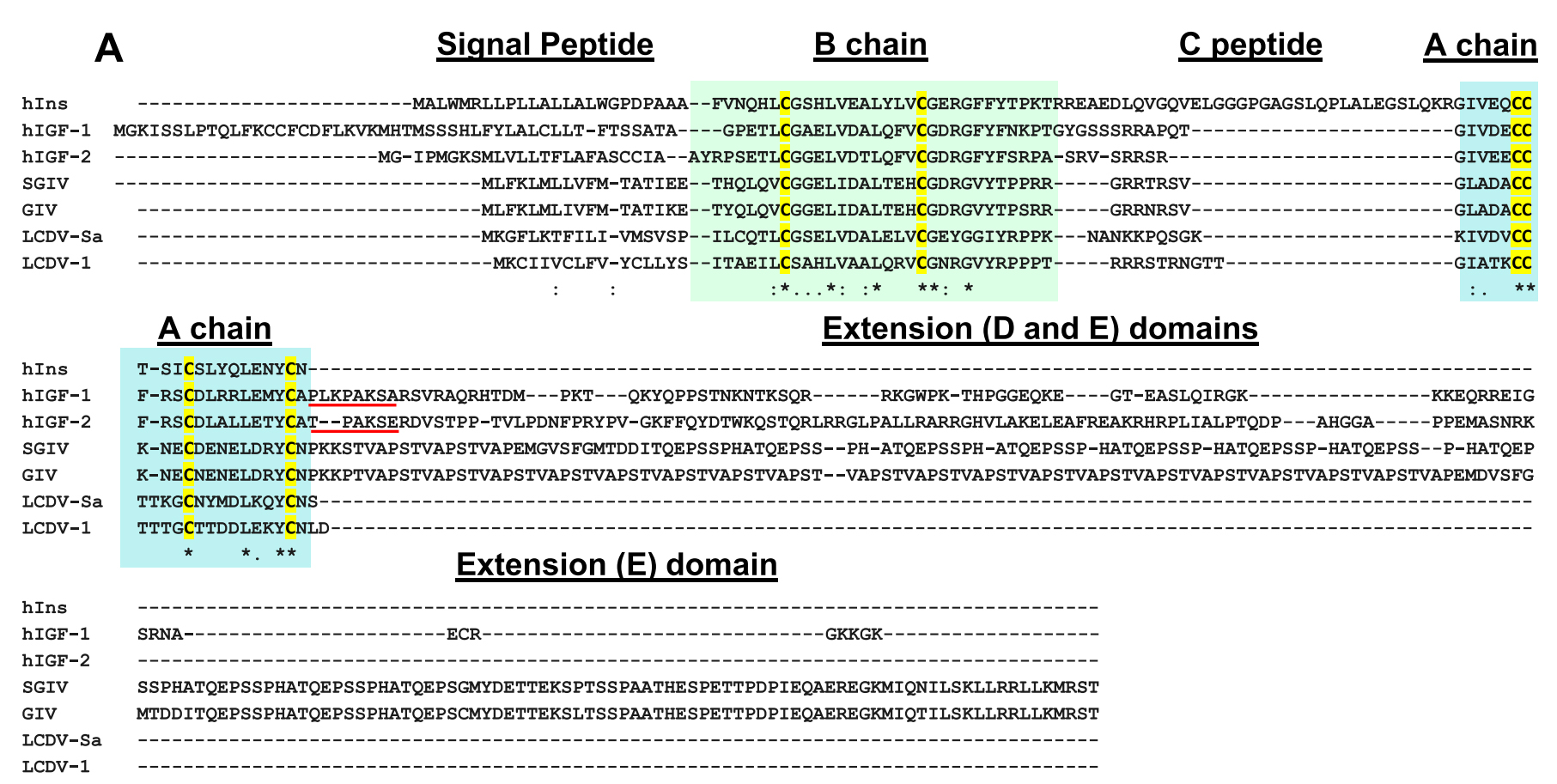
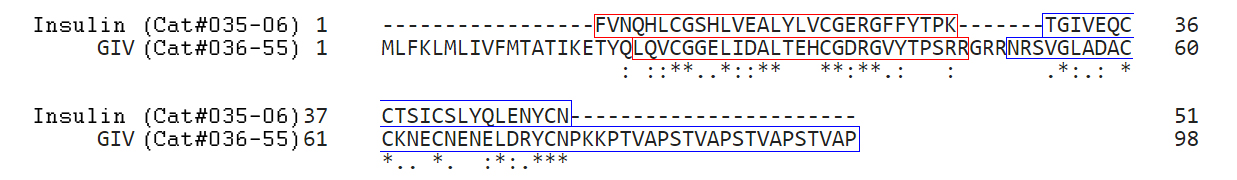
Objective: Members of the insulin/insulin-like growth factor (IGF) superfamily are well conserved across the evolutionary tree. We recently showed that four viruses in the Iridoviridae family possess genes that encode proteins highly homologous to human insulin/IGF-1. Using chemically synthesized single-chain (sc), i.e., IGF-1-like, forms of the viral insulin/IGF-1-like peptides (VILPs), we previously showed that they can stimulate human receptors. Because these peptides possess potential cleavage sites to form double chain (dc), i.e., more insulin-like, VILPs, in this study, we have characterized dc forms of VILPs for Grouper iridovirus (GIV), Singapore grouper iridovirus (SGIV) and Lymphocystis disease virus-1 (LCDV-1) for the first time.Methods: The dcVILPs were chemically synthesized. Using murine fibroblast cell lines overexpressing insulin receptor (IR-A or IR-B) or IGF1R, we first determined the binding affinity of dcVILPs to the receptors and characterized post-receptor signaling. Further, we used C57BL/6J mice to study the effect of dcVILPs on lowering blood glucose. We designed a 3-h dcVILP in vivo infusion experiment to determine the glucose uptake in different tissues.Results: GIV and SGIV dcVILPs bind to both isoforms of human insulin receptor (IR-A and IR-B) and to the IGF1R, and for the latter, show higher affinity than human insulin. These dcVILPs stimulate IR and IGF1R phosphorylation and post-receptor signaling in vitro and in vivo. Both GIV and SGIV dcVILPs stimulate glucose uptake in mice. In vivo infusion experiments revealed that while insulin (0.015 nmol/kg/min) and GIV dcVILP (0.75 nmol/kg/min) stimulated a comparable glucose uptake in heart and skeletal muscle and brown adipose tissue, GIV dcVILP stimulated 2-fold higher glucose uptake in white adipose tissue (WAT) compared to insulin. This was associated with increased Akt phosphorylation and glucose transporter type 4 (GLUT4) gene expression compared to insulin in WAT.Conclusions: Our results show that GIV and SGIV dcVILPs are active members of the insulin superfamily with unique characteristics. Elucidating the mechanism of tissue specificity for GIV dcVILP will help us to better understand insulin action, design new analogs that specifically target the tissues and provide new insights into their potential role in disease.
Chrudinová M, Moreau F, Noh HL, et al. Characterization of viral insulins reveals white adipose tissue-specific effects in mice. Molecular Metabolism. 2021;44:101121.
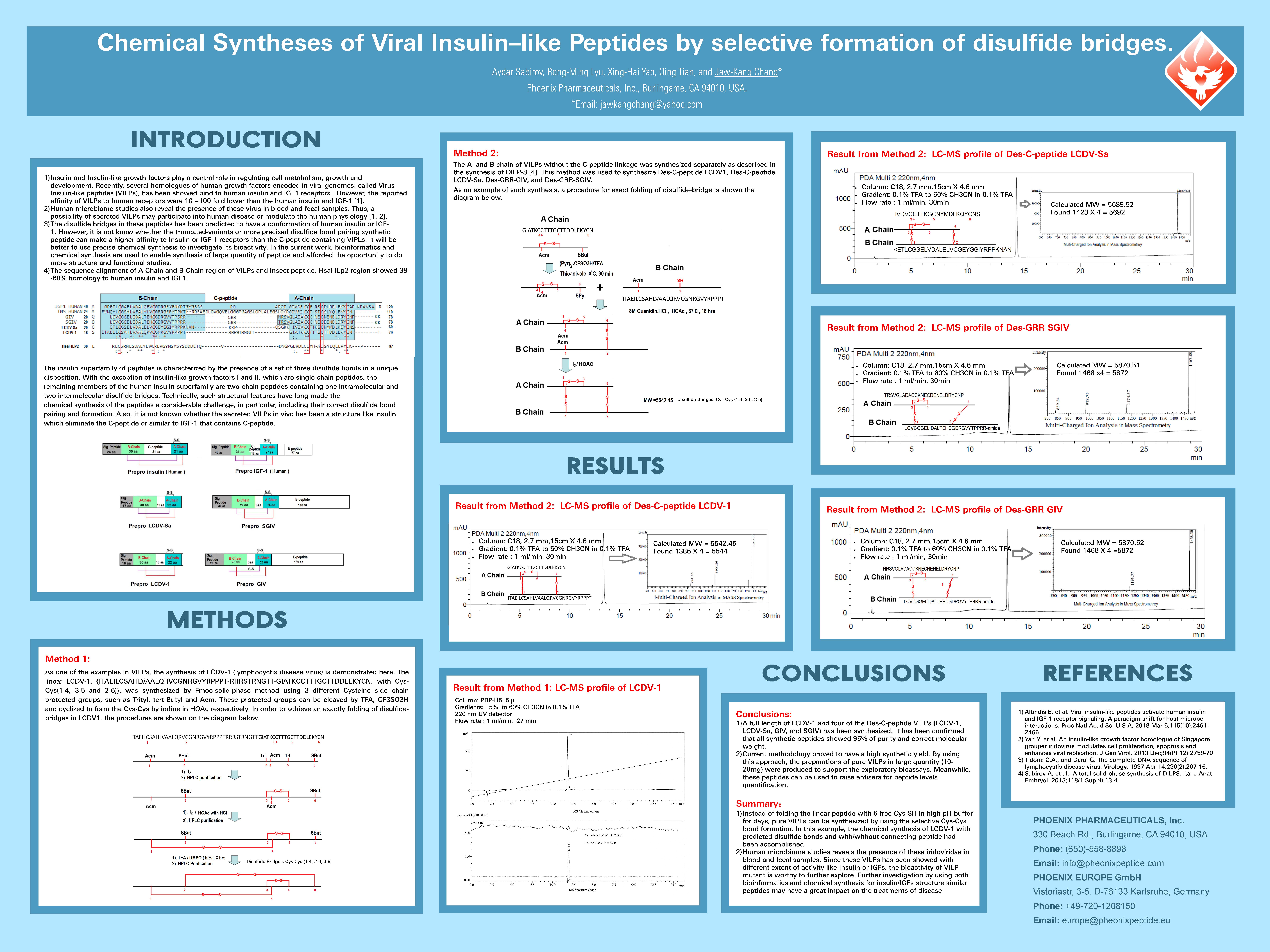
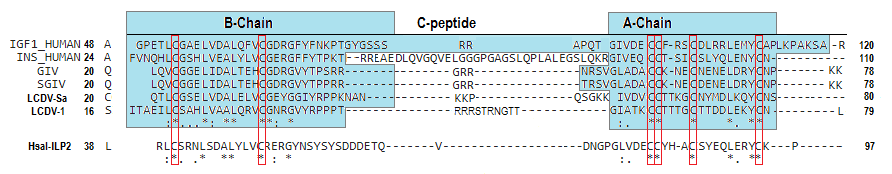
Viruses are the most abundant biological entities and carry a wide variety of genetic material, including the ability to encode host-like proteins. Here we show that viruses carry sequences with significant homology to several human peptide hormones including insulin, insulin-like growth factors (IGF)-1 and -2, FGF-19 and -21, endothelin-1, inhibin, adiponectin, and resistin. Among the strongest homologies were those for four viral insulin/IGF-1-like peptides (VILPs), each encoded by a different member of the family Iridoviridae VILPs show up to 50% homology to human insulin/IGF-1, contain all critical cysteine residues, and are predicted to form similar 3D structures. Chemically synthesized VILPs can bind to human and murine IGF-1/insulin receptors and stimulate receptor autophosphorylation and downstream signaling. VILPs can also increase glucose uptake in adipocytes and stimulate the proliferation of fibroblasts, and injection of VILPs into mice significantly lowers blood glucose. Transfection of mouse hepatocytes with DNA encoding a VILP also stimulates insulin/IGF-1 signaling and DNA synthesis. Human microbiome studies reveal the presence of these Iridoviridae in blood and fecal samples. Thus, VILPs are members of the insulin/IGF superfamily with the ability to be active on human and rodent cells, raising the possibility for a potential role of VILPs in human disease. Furthermore, since only 2% of viruses have been sequenced, this study raises the potential for discovery of other viral hormones which, along with known virally encoded growth factors, may modify human health and disease.
Emrah Altindis, Weikang Cai, Masaji Sakaguchi, et al, Proceedings of the National Academy of Sciences. 2018;:201721117.
Insulin-like growth factors (IGFs) play crucial roles in regulating cell differentiation, proliferation and apoptosis. In this study, a novel IGF homologue gene (IGF-like) encoded by Singapore grouper iridovirus (SGIV) ORF062R (termed SGIV-IGF), was cloned and characterized. The coding region of SGIV-IGF is 771 bp in length, with a variable number of tandem repeats (VNTR) locus at the 3'-end. We cloned one isoform of this novel gene, 582 bp in length, containing the predicted IGF domain and 3.6 copy numbers of the 27 bp repeat unit. SGIV-IGF was an early transcribed gene during viral infection, and SGIV-IGF was distributed predominantly in the cytoplasm with a diffused granular appearance. Intriguingly, overexpression of SGIV-IGF was able to promote the growth of grouper embryonic cells (GP cells) by promoting G1/S phase transition, which was at least partially dependent on its 3'-end VNTR locus. Furthermore, viral titre assay and real-time quantitative PCR (RT-qPCR) analysis proved that SGIV-IGF could promote SGIV replication in grouper cells. In addition, overexpression of SGIV-IGF mildly facilitated apoptosis in SGIV-infected non-host fathead minnow (FHM) cells. Together, our study demonstrated a novel functional gene of SGIV which may regulate viral replication and cellular processes through multiple mechanisms that appear to be cell type-dependent.
Yan Y, Cui H, Guo C, et al, J Gen Virol. 2013 Dec;94(Pt 12):2759-70. doi: 10.1099/vir.0.056135-0. Epub 2013 Sep 23.
Lymphocystis disease virus (LCDV) is the causative agent of lymphocystis disease, which has been reported to occur in over 100 different fish species worldwide. LCDV is a member of the family Iridoviridae and the type species of the genus Lymphocystivirus. The virions contain a single linear double-stranded DNA molecule, which is circularly permuted, terminally redundant, and heavily methylated at cytosines in CpG sequences. The complete nucleotide sequence of LCDV-1 (flounder isolate) was determined by automated cycle sequencing and primer walking. The genome of LCDV-1 is 102.653 bp in length and contains 195 open reading frames with coding capacities ranging from 40 to 1199 amino acids. Computer-assisted analyses of the deduced amino acid sequences led to the identification of several putative gene products with significant homologies to entries in protein data banks, such as the two major subunits of the viral DNA-dependent RNA polymerase, DNA polymerase, several protein kinases, two subunits of the ribonucleoside diphosphate reductase, DNA methyltransferase, the viral major capsid protein, insulin-like growth factor, and tumor necrosis factor receptor homolog.
Tidona CA, Darai G. Virology. 1997 Apr 14;230(2):207-16.

| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 036-55 | Des-GRR-GIV | 100 µg | $428 |
| 036-56 | Des-GRR-SGIV | 100 µg | $428 |
| 036-58 | Des-C-peptide-LCDV-1 / DesC-vPIF-1 | 100 µg | $428 |
| B-036-58 | Des-C-peptide-LCDV-1 / DesC-vPIF-1 - Biotin Labeled | 20 µg | $317 |
| T-036-58 | Des-C-peptide-LCDV-1 / DesC-vPIF-1 - I-125 Labeled | 10 µCi | $1082 |
| 036-61 | LCDV-1/ sc LCDV1-VILP / vPIF-1 | 100 µg | $490 |
| 036-59 | LCDV-Sa/ dc LCDV1-VILP | 100 µg | $428 |
| 036-54 | dcMFRV-VILP | 100ug | $408 |
| 036-57 | scMFRV-VILP | 100 µg | $497 |
Social Network Confirmation