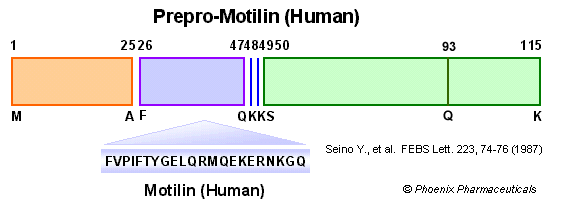
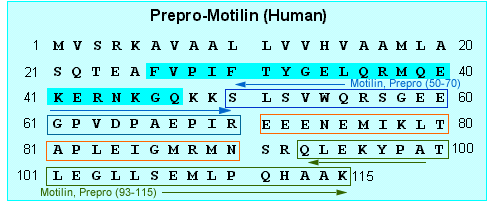
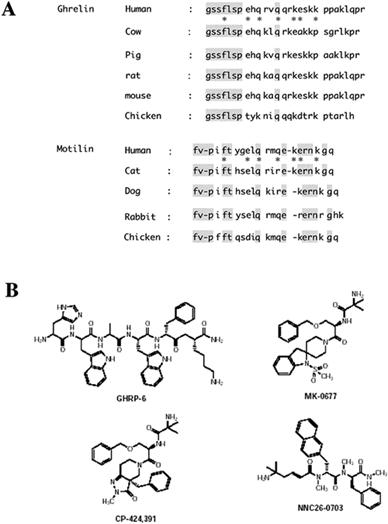
 |
Comparison of amino acid sequences of ghrelin and motilin (A) and chemical structures of growth hormone secretagogues (GHSs, B) Ghrelin is a 28 amino acid acyl-peptide esterified with octanoic acid on Ser 3. Des-[Gln14] ghrelin is produced from the alternative splicing of the peptide coding region. Ghrelin does not have a carboxyl-terminal amide group, but its acylation is required for activation of growth hormone secretagogue (GHS) receptor-1a, a G-protein-coupled receptor. Ghrelin and its receptor are highly conserved across species. Human ghrelin and motilin show 36% identity in amino acid residues. Similarly, the human ghrelin receptor (GHS receptor-1a) shows a remarkable 52% identity with the human motilin receptor (type 1a). Ghrelin and motilin thus represent a novel family of gastrointestinal hormones. MK-0677, CP-424,391, and NNC 26-0703 are examples of nonpeptidyl GHSs, which have been evaluated in long-term controlled clinical studies to assess their potential benefits in a variety of disease states such as idiopathic GH deficiency and catabolic states. |
AIMS: This study aimed to investigate: (i) the relation between fasting time and serum ghrelin, motilin and insulin concentrations and (ii) the correlations between these hormones and anthropometrical parameters of infants in the first 18 months of life.
PATIENT AND METHODS: A cross-sectional study on 62 term infants was performed. Blood samples for hormonal assay were obtained at least 1 h after feeding. Weight, length and head circumference were recorded. Plasma ghrelin, motilin and insulin concentrations were determined by radioimmunoassay.
RESULTS: Ghrelin and motilin had a significant direct correlation with fasting time (r = 0.447; P < 0.001 and r = 0.36; P = 0.004, respectively). We observed a negative influence of insulin on ghrelin levels (beta = -0.32; P = 0.036). Plasma ghrelin levels correlated significantly with age (r = 0.45, P < 0.001), weight (r = 0.31, P = 0.013), head circumference (r = 0.35, P = 0.006) and length (r = 0.39, P = 0.001). A significant correlation emerged between motilin and age (r = 0.45, P < 0.001), weight (r = 0.43, P = 0.001), head circumference (r = 0.47, P < 0.001) and length (r = 0.48, P < 0.001).
CONCLUSIONS: Fasting influence on serum ghrelin concentration confirms the role of this hormone as a physiological meal initiator also in infancy. The correlation between ghrelin, anthropometrical parameters and age supports the hypothesis that this hormone could exert an important influence on growth in the first months of life. Considering motilin, age and weight might play a role in determining its secretion; motilin could be considered one of the numerous factors involved in long-term regulation of energy balance.
Savino F, Grassino EC, Fissore MF, et al. Ghrelin, motilin, insulin concentration in healthy infants in the first months of life: relation to fasting time and anthropometry. Clin Endocrinol (Oxf). 2006;65(2):158-62.
Celiac disease (CD) is characterized by mucosal villous atrophy mostly confined to the proximal small intestine. Upper-gut motor abnormalities have been reported. Motilin, localized in cells in the proximal small intestine, is a trigger factor for the migrating motor complex. Plasma levels of motilin were studied in 16 untreated CD patients and in an age-matched control group of 18 healthy subjects by radioimmunoassay and by high-performance liquid chromatography (HPLC). The fasting levels of motilin and postprandial levels were significantly higher in CD patients compared to controls (P<0.01) and HPLC revealed a divergent individual pattern of the motilin fragments.
Sjölund K, Ekman R. Plasma motilin in untreated celiac disease. Peptides. 2003;24(3):483-6.
Motilin is secreted in a clear episodic pattern during fasting or during the interdigestive phase, but feeding promptly stops this secretory pattern, and plasma concentrations of motilin decrease.Intravenous (i.v.) injection of motilin (37 nmol/rat) suppressed LH release and significantly decreased mean LH concentrations both in ovariectomized (OVX) and oestradiol-implanted ovariectomized (OVX+E2) rats. Food intake was significantly increased by i.v. motilin injection in OVX rats, but not in OVX+E2 rats. It is likely that motilin inhibits LH release via inhibition of the gonadotrophin-releasing hormone (GnRH)-releasing mechanism at the hypothalamic level, because motilin (3.7 nmol/rat) also suppressed LH secretion when centrally administered, and because LH release in i.v. motilin-treated rats increased in response to exogenous GnRH. These results suggest that motilin may be a peripheral signal for the suppression of LH secretion through central sensors.
Tsukamura H, Tsukahara S, Maekawa F, et al. Peripheral or central administration of motilin suppresses LH release in female rats: a novel role for motilin. J Neuroendocrinol. 2000;12(5):403-8.
Motilin is a 22-amino acid peptide hormone expressed throughout the gastrointestinal (GI) tract of humans and other species. It affects gastric motility by stimulating interdigestive antrum and duodenal contractions. A heterotrimeric guanosine triphosphate-binding protein (G protein)-coupled receptor for motilin was isolated from human stomach, and its amino acid sequence was found to be 52 percent identical to the human receptor for growth hormone secretagogues. The macrolide antibiotic erythromycin also interacted with the cloned motilin receptor, providing a molecular basis for its effects on the human GI tract. The motilin receptor is expressed in enteric neurons of the human duodenum and colon. Development of motilin receptor agonists and antagonists may be useful in the treatment of multiple disorders of GI motility.
Feighner SD, Tan CP, Mckee KK, et al. Receptor for motilin identified in the human gastrointestinal system. Science. 1999;284(5423):2184-8.
|
|
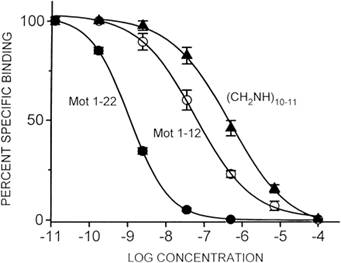 |
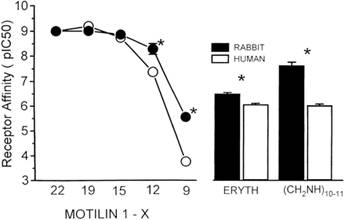
| From human antral mitochondrial fractions, hot saturation analysis and Scatchard analysis (inset), revealing a single binding site and a maximum motilin-binding capacity of 251.9 ± 27.9 fmol/mg. | Comparison of tissue affinity from rabbit or from human antrum for Mot-(1-22), Mot-(1-19), Mot-(1-12), Mot-(1-9), erythromycin (ERYTH), and Mot-(1-12) (CH2NH)10-11. * P < 0.001. |
 |
 |
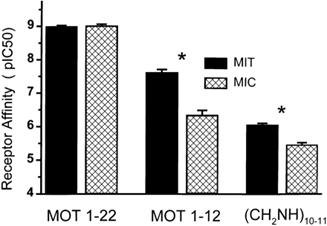
| Binding affinity of synaptosome-enriched mitochondrial fractions and of plasma membrane microsomal fractions for Mot-(122), Mot-(112), and Mot-(112) (CH2NH)10-11. * P < 0.001. |
Comparison of tissue affinity from rabbit or from human antrum for Mot-(122), Mot-(119), Mot-(112), Mot-(19), erythromycin (ERYTH), and Mot-(112) (CH2NH)10-11. * P < 0.001. |
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| RK-045-01 | Motilin (Canine) - RIA Kit | 125 tubes | $880 |
| EK-045-04 | prepro-Motilin (50-70) (Human) - EIA Kit | 96 wells | $570 |
| RK-045-03 | Motilin (Human, Porcine) - RIA Kit | 125 tubes | $880 |
| 001-29 | [Cys0]-GPR-38-A (390-412) / Motilin Receptor (Human) | 100 µg | $167 |
| 045-04 | prepro-Motilin (50-70) (Human) | 100 µg | $242 |
| H-045-04 | prepro-Motilin (50-70) (Human) - Antibody | 100 µl | $571 |
| B-045-04 | prepro-Motilin (50-70) (Human) - Biotin Labeled | 20 µg | $253 |
| T-045-04 | prepro-Motilin (50-70) (Human) - I-125 Labeled | 10 µCi | $1082 |
| G-045-04 | prepro-Motilin (50-70) (Human) - Purified IgG Antibody | 200 µg | $571 |
| RK-045-04 | prepro-Motilin (50-70) (Human) - RIA Kit | 125 tubes | $880 |
Social Network Confirmation